Neurofeedback for Optimal Performance
Human performance as a construct can be understood as a wide range of human capacity and associated self-directed purposeful physical and mental function. Graphic ©PRESSLAB/Shutterstock.com.
.jpg)
At one extreme is a person living with limited capacity and significant dysfunction (slow car). At the other extreme is a master athlete with enhanced capacity due to genetics and training (fast car).

There is little doubt that mitigating cellular damage by reducing toxins and enhancing cell function with nutrients, rest, and performance training leads to greater capacity and greater performance output. Age-related health and performance decline is also a known variable for which optimal brain and body performance training at any age is desired by professional athletes, corporate executives, and consumers alike. We won't address this method of optimizing peak performance; rather, we will address electrophysiology measures and manipulation to enhance performance.
One other attribute is the mechanisms underlying optimal performance over time or longevity. All human systems face age-related decline, including athleticism (Ganse et al., 2018). Military athletes exposed to unique and deleterious routines (i.e., sleeplessness, night shifts, high tempo engagements that maintain sympathetic arousal) can experience faster rates of decline as a result of mission-essential over-training and a more kinetic type of physical damage (i.e., parachute landings, very heavyweight loads on the musculoskeletal system and exposure to training or during deployment mission injury).

Unlike professional impact sports (i.e., MMA, football), military SOF athletes have far more exposure to high central nervous system stressors within a given month than a typical professional athlete. Also, more unique to military athletes are the critically essential maintenance of cognitive functions to use complex electronics and other equipment under high stress and fatigue conditions while maintaining a heightened situational awareness of potential threats.

BCIA Blueprint Coverage
This unit addresses V. Neurofeedback Optimal Performance Protocols. This unit covers Performance Attributes in qEEG, Culture and Goals of Neurofeedback in Performance, Evolution of Neurofeedback Protocols for Optimal Performance, Introduction to Alpha-Theta Training, Introduction to SMR Training, qEEG-Based NFB Implementation, Remote & Home-Use Performance Training, Monitoring Training, Measuring Progress, and Generalization to Field/Duty Performance, and Full Neurofeedback Session Demonstrations.
A. PERFORMANCE ATTRIBUTES IN qEEG

This section covers Performance Attributes in qEEG, including:
1) Focus and Attention,
2) Stress and Anxiety, and
3) Mood and Attitude.
Overview
Physical strength and flexibility remain essential attributes of optimal performance athletes, civilian or military. Graduated improvements in these areas are well understood and include proper rest, cell recovery, and nutritional support.When it comes to training using biofeedback and neurofeedback, optimal performance for civilian athletes and military athletes looks somewhat different than how it is applied to a clinical population.
Wilson and Peper (2011) observed that while patients seek biofeedback to reduce adverse symptoms, athletes seek the technology to optimize performance. They also recognized that there are baseline differences between athletes and non-athletes.
Athletes often differ from typical patients. Male and female athletes resemble each other more than they resemble the sex norms of the average population on a wide variety of measures. They are motorically, psychologically, and anatomically different as their underlying brain mechanisms and structures are different. At this time, it is unknown whether these differences are due to genetics, early learning, or extensive practice. Most likely they are the result of an ongoing interaction of these three variables. (Wilson & Peper, 2011).
Focus and Attention
One of the more important cognitive skills essential for military athletic performance is the capacity to engage and maintain focus and attention to detail when it matters.The first step is to properly measure the baseline or current brain state related to focus and attention capacity. Focus and attention to “what” matters too. Part of attention to detail involves the sensory processing functional capacity. The brain’s sensory system involves input from both external (extra-receptors) and internal to the body (intra-receptors) stimuli (e.g., pain, heat, touch, smell, sound).
EEG recordings are perhaps the best and least invasive way to reliably evaluate the brain's capacity to orient or attend to stimuli.
Two primary methods are event-related potentials and EEG/qEEG measurement of frequency values. First, we can measure event-related potentials (ERPs) specific to the function and region of cortical involvement in that function.
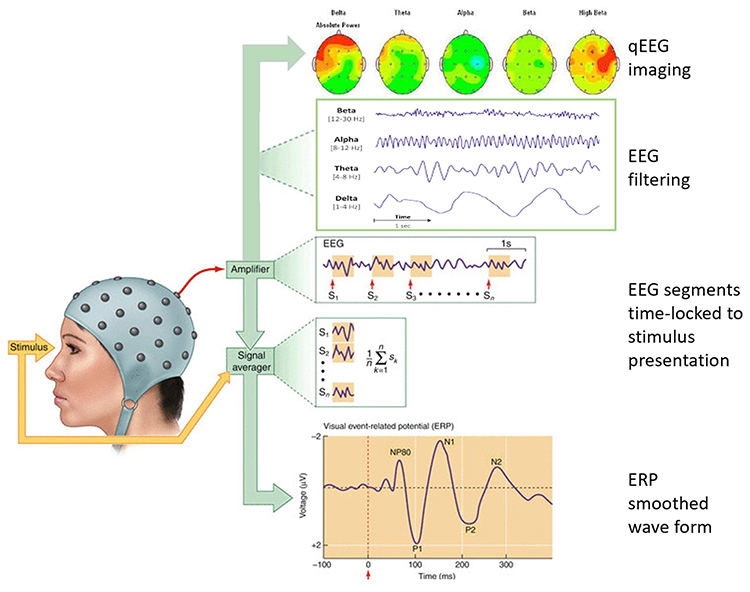
Second, we can measure EEG/qEEG frequency values at cortical regions of interest and calculate those values against a reference group.
Event-Related Potentials: Basic Principles
When a person is exposed to a stimulus (e.g., a flash of light or a movement off to the side of the visual field or an atypical sound), the brain does something called desynchronization. This can be seen by a fast and brief suppression of EEG frequencies in the alpha and beta bands. It appears that these EEG frequencies are involved in the processing or modulation of information flow along neural networks.It is common to record sensory processing speed using ERPs, where a sensory stimulus is presented, and the change post-stimulus delivery can be quantified from the recording EEG. More complex mental processes, or higher-order cognition, are also measured with ERP. For peak performance applications, the value of rapid sensory responses and, likewise, rapid cognitive processing of information can measure baseline function and improvements with training (e.g., react to contact drills).
Event-Related Potentials: Sensory N100
In this example of auditory processing to a white noise sound (recorded at CZ), you will see a baseline measure (black line) followed by a post measure (blue line) after induced fatigue. The deep fatigue resulted in a reduced amplitude and delayed or longer latency. While we call this component N100, you will note that the waveform is closer to N200.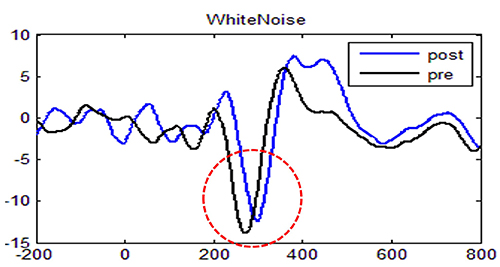
The complexity of the stimulus used to produce the component matters. You cannot compare a high-frequency beep tone to a longer-duration white noise sound. Each produces different ERP components.
The difference may be trivial for clinical applications, but for athletic performance or reaction to contact scenarios, 50 milliseconds makes a difference.
In this example of visual attention to one's external surroundings (black and white checkerboard stimulus, sensor recording at CZ), you will see a baseline measure (black line) followed by a post measure (pink line) after induced fatigue. The deep fatigue resulted in a negligible amplitude and latency difference. While we call this component P300a, you will note that the positive amplitude peak is closer to 500ms. Different tasks or stimulus presentation types will result in differing average latency and amplitude. Normal latency is also age- and gender-sensitive. This is why stimulus types are consistently used when comparing performance improvements.
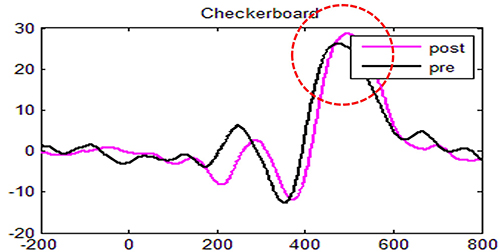
The P300a component is dopamine-mediated and reflects frontal working functions that are not only associated with attention and focus but also indirectly involved in memory. If a person cannot attend to external environmental stimuli, it is less likely that the information will be available for further processing of that data into memory.
In the context of mind-body integration, Harvard Medical School sports psychiatrist Eric Leskowitz, MD (2017) wrote, “Consider an application: the observed 37 msec decrease in reaction time would enable a baseball player to perceive a 90 mph fastball as though it was only traveling 80 mph. A key feature of the flow state is the sense of time slowing down or stopping” (p. 1).
ERPs and continuous performance task measures provide objective millisecond measures to evaluate and monitor peak performance (Mirifar et al., 2019). The number of training sessions is as important as the type of training (protocol) for neurofeedback (Domingos et al., 2021). Less than a dozen trials, especially if mixed with differing protocols, are less likely to yield large enough cortical changes where “the difference, really makes a difference.”
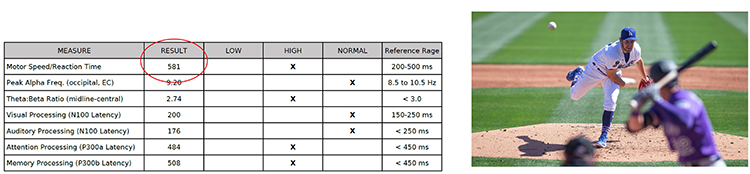
Case Example

Graphic © Mike Orlov/Shutterstock.com
GOAL: In a case study of a professional boxer, nutritionists and sports psychologists incorporated neurofeedback to help the athlete gain focus and improve impulse control (Larson et al., 2012).METHOD: The athlete completed 25 neurofeedback sessions designed to suppress slower frequency EEG power while increasing beta1 frequencies in the frontal and bilateral temporal locations. An additional 17 sessions were completed to inhibit the power of the faster EEG frequencies and augment slow-mid spectrum frequencies recorded from the parietal scalp locations.
RESULT: The result or target of success from the intervention was that the boxer was able to experience a sense of heightened focus yet with a calmness of mind.
Stress and Anxiety
Cognitive or mental strength and flexibility are also becoming better understood and differentiate between amateur athletes and those who excel at the professional level.In a 2014 study of amateur and professional athletes, qEEG results identified that posterior and occipital region alpha frequency band power was lower than typical when experiencing performance anxiety, both for armature and professional athletes (Tharawadeepimuk & Wongsawat, 2014).
However, when it was time for fully-engaged competition, only the professional athlete showed a return to normal alpha power.
Motor and cognitive performance have been linked with EEG frequencies in the alpha band, making it a reasonable neurofeedback target to optimize performance (Mottaz et al., 2015).
With as little as seven sessions of alpha coherence neurofeedback applied over healthy subjects' motor cortex, the results were lasting and improved hand motor performance (Mottaz et al., 2015).
Beyond motor reaction time and calmness under stressful conditions, the ability of the central nervous system to remain capable of continued learning and detailed recall, alpha peak training has also been reported to aid memory functions.
Memory is not simply a critical cognitive function for older adults and laypeople. Military athletes need to perform well in advancing and complex use of battlefield equipment. The additional use of memory enhancement, even related to language learning, is an important and underrecognized optimal performance target for neurofeedback (Nan et al., 2012)..
The thalamic generation of alpha has been implicated in anxiety. Theta/alpha ratio neurofeedback effectively modulates this important stress-response function (Zadkhish et al., 2017).
In a study of male soccer players, 12 alpha/theta neurofeedback sessions at scalp location PZ were provided. Compared to the control group, the neurofeedback group demonstrated reduced performance anxiety and enhanced athletic performance (Tharawadeepimuk & Wongsawat, 2016).
The Kim’s Game (keep-in-memory-system) has been around over 100 years and is utilized among military trainers to enhance situational awareness and several memory functions. It is applied under stress conditions and without, all to aid in the improved cognitive processing and memory of those that need recall accuracy following brief observation periods. Training memory skills under stress is done to train the brain to process only the relevant sensory information and suppress irrelevant information.

One frequently targeted brain region related to the application of attention to detail while under stress is the anterior cingulate cortex (ACC). The anterior cingulate cortex (ACC) if often a target of neurofeedback training for several reasons. The ACC is heavily involved in the ability to monitor actions and the results of actions from which adjustments could be made. The ACC is therefore involved in decision making, a critical skill on the fast-moving sports arena or the battlefield.
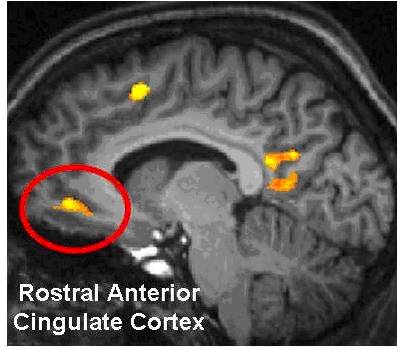
The ACC has structural divisions that are loosely referred to as “parts” and collectively serve our function of monitoring the environment. These are the motor input with connections to all motor areas in the brain, the affective input (called the limbic part) influenced by the amygdala and thalamus. The affective input (called the limbic part) is influenced by the amygdala and thalamus. Most dorsally in the ACC is the cognitive part.
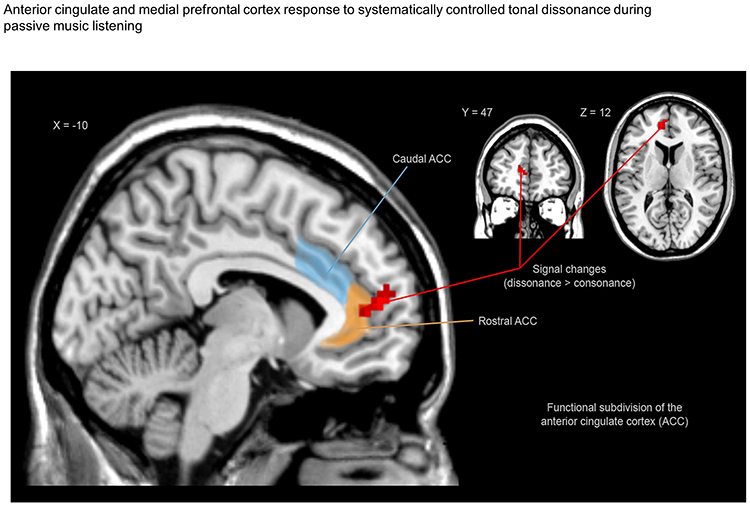
According to the theory proposed by Bush and collaborators (Bush et al., 2000), the ACC presents a functional division in which caudal‐dorsal regions (blue color) serve a variety of cognitive functions, whereas rostral‐ventral regions are involved in emotional tasks (orange color). The red color indicates fMRI results, sagittal view showing stronger signals during dissonance (compared to consonance) in the left medial prefrontal cortex (mPFC) and the left anterior cingulate cortex (ACC).
Neurofeedback applications have been specifically developed for athletes by capitalizing on this important and multi-function ACC brain region, and may well represent the more commonly modulated region of the brain among neurofeedback providers and consumer-facing protocols. Single sensor/electrode training over CZ or FZ has alone been shown to be very effective in the training of attention, focus, and anxiety.
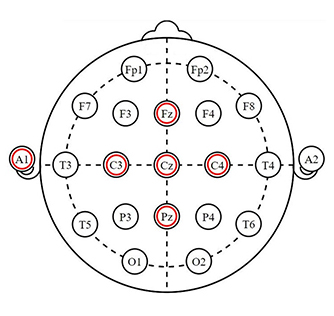 |
 |
By suppressing elevated beta2 frequency power, one may see reduced anxiety expression. Commonly, this is trained in conjunction with heart rate variability biofeedback.
With the reduction of slow content (theta) dominance in the EEG over the ACC, one may see improved focus and attention.
With ACC function training, event-related potentials are often used to record the orienting response, a function of awareness. Specifically, the P300a and P400, that occur under the “NoGo” stimulus condition, serve to show the number of milliseconds the brain takes to process this monitoring function.
Mood and Attitude
Affect, another word for “mood,” is also connected to the ACC as was discussed within the context of limbic system input.
Mood is a conscious awareness of one's affective or emotional state. It adds a dimension to our reasoning and decision-making and, when imbalanced, will also reduce functions of attention or focus. Basic emotions include anger, fear, joy, sadness, disgust, ands surprise. More complex are conditions of sympathy, guilt, admiration, and contempt. Most everyone’s brain is wired to seek positive emotions and avoid difficult emotions. In some instances, this sets up a motivating condition where behaviors like excessive alcohol consumption temporarily modulate emotions in a more desirable direction.
In general, the limbic lobe, in synchrony with the hypothalamus and amygdala, has been described as serving the role of mood or emotional states. This is not to say that mood and emotion are limited to these structures. Rather, the system that produces affect involves cortical and non-cortical interconnections that are responsive to internal and external stimuli.
Moods and attitudes that are challenging carry terms like depression, sadness, worry, and anxiety. Moods and attitudes that are positive carry terms like joy, contentment, and happiness.
Both mood and attitude states are in part regulated by the brain’s monitoring capacity, with the ACC being a contributing structure that is readily accessed for modulation with neurofeedback, the from anterior regions in particular (F3, FZ, F4).
For many years, the dorsolateral prefrontal cortex (DLPFC) was determined to be a more dominant location of the frontal lobes related to mood. Specifically, if there was hypofunction in the left frontal region of interest, depressed or flat mood was a likely correlation. Some researchers in the 1990s found a correlation between depression and frontal alpha asymmetry (FAA), which is where the left frontal (F3) alpha activity was greater than that on the right (F4).
There does seem to be a relationship with hypofunction among the left DLPFC. Still, research by Dr. Martijn Arns has convincingly demonstrated that an alpha asymmetry score is diagnostically insufficient as a single ratio value (van der Vinne et al., 2017). Of note, the FDA approved rTMS 10Hz neurostimulation to the left DLPFC for Major Depressive Disorder and Bipolar Disorder based on strong clinical evidence. As seen by mixed results of left DLPFC 10Hz rTMS, the different subtypes of depressed mood are evidenced by different EEG patterns beyond just FAA.
Regarding peak performance, improved mood is correlated with improved focus, sleep recovery, and overall optimized function. Neurofeedback has been applied successfully using different frequency and scalp placement protocols (Lee et al., 2019; Linden, 2014).
Subjective explanations from those that train the brain with NFK report improved control over thought processes and mood regulation. It is this more subjective expression, seen in behavior and carried over to motivation to excel, that we find “attitude” to be relevant for optimal performance. Because affect is so tightly related to attitude, it is often a target of neurofeedback.
Mindfulness is a key pathway towards improved mood and attitude. Those who practice mindfulness meditation report feeling more “clear-headed” and able to perform without hindering self-judgment (Birrer et al., 2012; Tingaz & Çakmak, 2021).
The main asymmetry-based EEG-NF protocol has used an asymmetry index of alpha power as a feedback signal and trained patients to increase the right-to-left ratio, essentially rebalancing a putative hypoactivation of the left hemisphere. This asymmetry index is computed as A=100x(R-L)/R+L), where R and L are the square root of the power of alpha activity (obtained by Fast Fourier Transformation) measured at a right and left frontal electrode, respectively.
Meditation training has been found to influence alpha and theta frequency bands and modulate the Default Mode Network, a brain network instrumental in quality sleep and anxiety management (Nicholson et al., 2020). In particular, slower alpha (alpha1), theta modulation, and reduced beta frequency power are reported with hypnosis and meditation.
Occipital alpha power increases with meditation training and alpha training to increase and decrease power using MRI-neurofeedback has been used (personal communication with Dr. Ruth Lanius) to reduce symptoms of PTSD. The influence of alpha neurofeedback and meditation on the DMN is experiences in what appears to be a strengthening of one’s “self-referential” thoughts and attitude. This is the brain’s process of taking in external stimuli from around you and seeing yourself in that context. It is a more self-reflective capacity that can temper attitude and mood.
LORETA has been used to define brain regions of interest related to both neurofeedback and meditation. The use of LORETA with meditation reflects that beta is reduced within the ACC, and theta/alpha is enhanced in the posterior cingulate cortex (PCC), cuneus and precuneus. The use of neurofeedback at central midline locations (Fz, Cz, Pz) permits these frequencies and locations within the DMN to likewise be modulated. The result is potential mood stability through greater self-regulation capacity (Braboszcz & Delorme, 2011; Braboszcz et al., 2010; Brandmeyer & Delorme, 2013; Cahn et al., 2013; DeLosAngeles et al., 2016; Deolindo et al., 2020; Fingelkurts et al., 2016; Josipovic, 2010; Lazar et al., 2005; Lomas et al., 2015; Lutz et al., 2004; Travis & Parim, 2017; Zhigalov et al., 2019).
Alpha and theta neurofeedback share many similarities with mindfulness meditation with the advantage of objective EEG measures with neurofeedback. Both modalities facilitate improved concentration and emotional regulation.
Emotional regulation is considered a frontal lobe function and behaviorally can also be characterized as a facet of attitude. Learning to gain cognitive control with either neurofeedback or meditation has direct peak performance benefits.
References
Birrer, D., Röthlin, P., & Morgan, G. (2012). Mindfulness to enhance athletic performance: Theoretical considerations and possible impact mechanisms. Mindfulness, 3(3), 235–246. https://doi.org/10.1007/s12671-012-0109-2
Braboszcz, C., & Delorme, A. (2011). Lost in thoughts: Neural markers of low alertness during mind wandering. NeuroImage, 54(4), 3040–3047. https://doi.org/10.1016/j.neuroimage.2010.10.008
Braboszcz, C., Hahusseau, S., & Delorme, A. (2010). Meditation and neuroscience: From basic research to clinical practice. In R. Carlstedt (Ed.), Integrative clinical psychology, psychiatry and behavioral medicine: Perspectives, practices and research. Springer Publishing.
Brandmeyer, T., & Delorme, A. (2013). Meditation and neurofeedback. Frontiers in psychology, 4, 688. https://doi.org/10.3389/fpsyg.2013.00688
Bush, G., Luu, P., & Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex, Trends in Cognitive Sciences, 4(6), 215-222. https://doi.org/10.1016/S1364-6613(00)01483-2
Cahn, B. R., Delorme, A., & Polich, J. (2013). Event-related delta, theta, alpha and gamma correlates to auditory oddball processing during Vipassana meditation. Social Cognitive and Affective Neuroscience, 8(1), 100–111. https://doi.org/10.1093/scan/nss060
DeLosAngeles, D., Williams, G., Burston, J., Fitzgibbon, S. P., Lewis, T. W., Grummett, T. S., Clark, C. R., Pope, K. J., & Willoughby, J. O. (2016). Electroencephalographic correlates of states of concentrative meditation. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 110, 27–39. https://doi.org/10.1016/j.ijpsycho.2016.09.020
Deolindo, C. S., Ribeiro, M. W., Aratanha, M. A., Afonso, R. F., Irrmischer, M., & Kozasa, E. H. (2020). A critical analysis on characterizing the meditation experience through the electroencephalogram. Frontiers in Systems Neuroscience, 14, 53. https://doi.org/10.3389/fnsys.2020.00053
Domingos, C., & Peralta, M., Prazeres, P., Nan, W., Rosa, A., & Pereira, J. (2021). Session frequency matters in neurofeedback training of athletes. Applied Psychophysiology and Biofeedback, 46(2), 195-204. https://doi.org/10.1007/s10484-021-09505-3
Fingelkurts, A. A., Fingelkurts, A. A., & Kallio-Tamminen, T. (2016). Long-term meditation training induced changes in the operational synchrony of default mode network modules during a resting state. Cognitive Processing, 17(1), 27–37. https://doi.org/10.1007/s10339-015-0743-4
Ganse, B., Ganse, U., Dahl, J., & Degens, H. (2018). Linear decrease in athletic performance during the human life span. Frontiers in Physiology, 9, 1100. https://doi.org/10.3389/fphys.2018.01100
Josipovic Z. (2010). Duality and nonduality in meditation research. Consciousness and cognition, 19(4), 1119–1123. https://doi.org/10.1016/j.concog.2010.03.016
Larson, N. C., Sherlin, L., Talley, C., & Gerais, M. (2012). Integrative approach to high-performance evaluation and training: Illustrative data of a professional boxer. Journal of Neurotherapy, 16(4), 285-292. https://doi.org/10.1080/10874208.2012.729473
Lazar, S. W., Kerr, C. E., Wasserman, R. H., Gray, J. R., Greve, D. N., Treadway, M. T., McGarvey, M., Quinn, B. T., Dusek, J. A., Benson, H., Rauch, S. L., Moore, C. I., & Fischl, B. (2005). Meditation experience is associated with increased cortical thickness. Neuroreport, 16(17), 1893–1897. https://doi.org/10.1097/01.wnr.0000186598.66243.19
Lee, Y. J., Lee, G. W., Seo, W. S., Koo, B. H., Kim, H. G., & Cheon, E. J. (2019). Neurofeedback treatment on depressive symptoms and functional recovery in treatment-resistant patients with major depressive disorder: An open-label pilot study. J Korean Med Sci., 34(42), e287. https://doi.org/10.3346/jkms.2019.34.e287
Leskowitz, E. (2017). The zone: A measurable (and contagious) exemplar of mind–body integration. The Journal of Alternative and Complementary Medicine, 23(5), pp. 324–325. http://dx.doi.org/10.1089/acm.2017.29023.ejl
Linden D. E. J. (2014). Neurofeedback and networks of depression. Dialogues Clin Neurosci., 16(1), 103–112. https://doi.org/10.31887/DCNS.2014.16.1/dlinden
Lomas, T., Ivtzan, I., & Fu, C. H. (2015). A systematic review of the neurophysiology of mindfulness on EEG oscillations. Neuroscience and Biobehavioral Reviews, 57, 401–410. https://doi.org/10.1016/j.neubiorev.2015.09.018
Lutz, A., Greischar, L. L., Rawlings, N. B., Ricard, M., & Davidson, R. J. (2004). Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proceedings of the National Academy of Sciences of the United States of America, 101(46), 16369–16373. https://doi.org/10.1073/pnas.0407401101
Mirifar, A., Keil, A., Beckmann, J., & Ehrlenspiel, F. (2019). No effects of neurofeedback of beta band components on reaction time performance. J Cog Enhanc, 3, 251-260. https://doi.org/10.1007/s41465-018-0093-0
Mottaz, A., Solcà, M,, Magnin, C., Corbet, T., Schnider, A., & Guggisberg, A. G. (2015). Neurofeedback training of alpha-band coherence enhances motor performance. Clinical Neurophysiology, 126(9), 1754-1760. https://doi.org/10.1016/j.clinph.2014.11.023
Nan, W., Rodrigues, J. P., Ma, J., Qu, X., Wan, F., Mak, P. I., Mak, P. U., Vai, M. I., & Rosa, A. (2012). Individual alpha neurofeedback training effect on short term memory. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 86(1), 83–87. https://doi.org/10.1016/j.ijpsycho.2012.07.182
Nicholson, A. A., Ros, T., Densmore, M., Frewen, P. A., Neufeld, R., Théberge, J., Jetly, R., & Lanius, R. A. (2020). A randomized, controlled trial of alpha-rhythm EEG neurofeedback in posttraumatic stress disorder: A preliminary investigation showing evidence of decreased PTSD symptoms and restored default mode and salience network connectivity using fMRI. NeuroImage: Clinical, 28, 102490. https://doi.org/10.1016/j.nicl.2020.102490
Tharawadeepimuk, K. & Wongsawat, Y. (2014). QEEG evaluation for anxiety level analysis in athletes. The 7th 2014 Biomedical Engineering International Conference, 1-4. http://dx.doi.org/10.1109/BMEiCON.2014.7017400
Tharawadeepimuk, K. & Wongsawat, Y. (2016). QEEG coherence evaluation for soccer performance level analysis of the striker. In R. Wang R. & X. Pan (Eds.). Advances in cognitive neurodynamics. Springer. https://doi.org/10.1007/978-981-10-0207-6_51
Tingaz, E.O., & Çakmak, S. (2021). Mindfulness, self-compassion, and athletic performance in student-athletes. J Rat-Emo Cognitive-Behav Ther. https://doi.org/10.1007/s10942-021-00434-y
Travis, F., & Parim, N. (2017). Default mode network activation and Transcendental Meditation practice: Focused Attention or Automatic Self-transcending?. Brain and Cognition, 111, 86–94. https://doi.org/10.1016/j.bandc.2016.08.009
van der Vinne, N., Vollebregt, M. A., van Putten, M. J. A. M., & Arns, M. (2017). Frontal alpha asymmetry as a diagnostic marker in depression: Fact or fiction? A meta-analysis. NeuroImage: Clinical, 16, 79-87. https://doi.org/10.1016/j.nicl.2017.07.006
Wilson, V., & Peper, E. (2011). Athletes are different: Factors that differentiate biofeedback/neurofeedback for sport versus clinical practice. Biofeedback, 39(1), 27–30. https://doi.org/10.5298/1081-5937-39.1.01
Zadkhosh, S. M., Zandi, H. G., & Hemayattalab, R. (2017). The effects of Neurofeedback on anxiety decrease and athletic performance enhancement. Journal of Applied Psychological Research, 7(4), 21-37. https://doi.org/10.22059/japr.2017.61078
Zhigalov, A., Heinilä, E., Parviainen, T., Parkkonen, L., & Hyvärinen, A. (2019). Decoding attentional states for neurofeedback: Mindfulness vs. wandering thoughts. NeuroImage, 185, 565-574. https://doi.org/10.1016/j.neuroimage.2018.10.014
B. CULTURE AND GOALS OF NEUROFEEDBACK IN PERFORMANCE
NFB offers several methods or types, but they are all designed to deliver changes to the brain networks through EEG regulation. Examples include LORETA Brodmann targeting z-score, select frequency range amplitude, frequency, and site selected coherence. Each method is designed to alter behaviors. Examples are covert thoughts and self-reflection, mood stability, motor processing speed, anxiety reduction, memory, and a combined relaxation with increased focus/attention. The end result is a more efficient brain and nervous system equipped to meet performance demands (athletic skills, shooting accuracy, restful sleep) through increased neuroplasticity resulting in a greater capacity to self-regulate one's internal cognitive state.
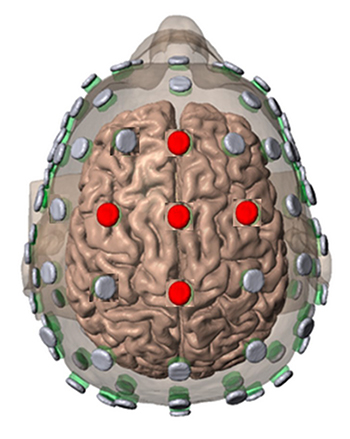
Frequency and Location for Neurofeedback for Performance (Anmin et al, 2021; Doppelmayr et al., 2008; Xiang et al., 2018).
SMR (C3, CZ, C4) 12-15Hz enhance while inhibiting theta 3-7Hz and beta 18-30Hz
Frontal Alpha reduction (8-12Hz)
Frontal theta reduction
Frontal, Central, Parietal Beta reduction
Alpha/Theta Crossover (PZ, OZ)
References
Doppelmayr, M., Finkenzeller, T., & Sauseng, P. (2008). Frontal midline theta in the pre-shot phase of rifle shooting: differences between experts and novices. Neuropsychologia, 46(5), 1463–1467. https://doi.org/10.1016/j.neuropsychologia.2007.12.026
Gong, A., Gu, F., Nan, W., Qu, Y., Jiang, C., & Fu, Y. (2021). A review of neurofeedback training for improving sport performance from the perspective of user experience. Frontiers in Neuroscience, 15, 638369. https://doi.org/10.3389/fnins.2021.638369
Xiang, M.-Q., Hou, X.-H., Liao, B.-G., Liao, J.-W., & Hu, M. (2018). The effect of neurofeedback training for sport performance in athletes: A meta-analysis. Psychology of Sport and Exercise, 36, 114-122. https://doi.org/10.1016/j.psychsport.2018.02.004.
C. EVOLUTION OF NEUROFEEDBACK PROTOCOLS FOR OPTIMAL PERFORMANCE

This section covers Early Protocols (Training Protocols, Defining an Effective Training Protocol, and Primary Schools of Thought in Neurofeedback Training) and Selecting a Protocol Model.
Overview
The development of training protocols in neurofeedback has been the result of an evolution of training practices, some growing out of early observational results related to research studies and some developing from attempts to elicit responses in the human or animal EEG that were either atypical or were an attempt to enhance already existing characteristics. This section will explore the history of this evolution and some of the significant milestones that have guided neurofeedback clinical applications during its 60+ year history.
The term protocol refers to a procedure for teaching individuals to achieve proficiency through instruction and practice. This definition works quite well for neurofeedback training and is an excellent description of the process. Neurofeedback training is a training process that utilizes reinforcement and instruction to enable an individual to develop improved proficiency or skill in a particular cognitive, mental or central nervous system activity-related task. Graphic courtesy of BrainMaster.com.

Generally accepted definitions of neurofeedback suggest that it is a process of operant conditioning leading to self-regulation of brain activity. Marzbani and colleagues (2016) state that “Neurofeedback is a kind of biofeedback, which teaches self-control of brain functions to subjects by measuring brain waves and providing a feedback signal.”
However, Strehl (2014) wrote that the learning process associated with neurofeedback requires more than operant conditioning and simple feedback. We also need to understand the influence of classical conditioning, skill learning, and motivational aspects.

She noted that all types of learning, including neurofeedback, result from trial and error, conscious and unconscious responses to events, including feedback and reinforcement, and an awareness of the results of such efforts. To facilitate the ability to incorporate these new skills into everyday life, known as generalization, we need to incorporate a behavioral therapy approach to the process of neurofeedback training. Therefore, our analysis of the development of training protocols will include evaluating which protocols may more effectively meet the client's needs in light of the learning requirements noted above.
Quantitative EEG
Quantitative EEG methods include the ability to compare EEG recordings to reference database samples. Some reference groups are considered “normal” while others used are a sample of patients that may not be considered a good reference since by definition they are seeking medical care assumably for brain related conditions. Other reference groups consist of special operations military groups or sports teams, in other words are peer-reference groups from which to compare.Having a reference group allows for comparison statistics like z-scores. Most all professional use neurofeedback software can now permit any number of frequency bands or all frequencies to be modulated in the direction of a z-score of 0. The z-scores can represent frequency amplitude or coherence values (Thatcher et al., 2020). Graphic © Peter Hermes Furian/Shutterstock.com.
LORETA and Brodmann Area Training
This approach can also include z-score measures and may in some cases be of some advantage over surface EEG neurofeedback. LORETA-guided neurofeedback allows a degree of focality to brain regions of interest (e.g., Brodmann Areas) to be the target of the feedback. The only additional consideration is that LORETA calculations require upwards of at least 16 scalp sensor connections, whereas surface z-score training can use a single active channel location (e.g., CZ). Individual athletes may have a specific area of interest to enhance, and this can be achieved with LORETA. Graphic © Sinauer.
Evolution of Neurofeedback Training Protocols for Optimal Performance
Difficulties with establishing the efficacy of a particular behavioral intervention have been covered in the Research Evidence Basis for Neurofeedback section. However, it is helpful to note that this review article (Monderer et al., 2002) cites multiple research studies that utilized treatments other than the sensorimotor rhythm training, including slow cortical potential training, training to inhibit epileptiform activity in the EEG and several other techniques. They also did not evaluate specific training approaches and whether interventions were applied appropriately. In light of Strehl (2014), it appears clear that simply using operant conditioning techniques without appropriate additional components noted in her paper may result in reduced effectiveness for otherwise helpful treatment.
In an attempt to elucidate the factors associated with effective neurofeedback training, Rogala and colleagues (2016) identify some of the do's and don’ts of effective neurofeedback training. Included in the list of approaches with positive effects is using more specific electrode locations chosen to be associated with known sources of the particular EEG frequency activity being trained, for example, frontal midline theta or posterior alpha activity.
The use of multiple electrodes in the general area identified as a source of this activity is also recommended to represent the source areas more completely. They suggest that neurofeedback trainers and researchers should identify optimal training electrode locations based on anatomical and functional studies and develop protocols that may use a weighted average approach for the input from various electrode locations.
Suggestions from the same paper of what not to do include avoiding training multiple EEG frequencies simultaneously to avoid confusion and cross-frequency interactions that may result in effects other than those desired for the training protocol. This suggestion did not have much basis in research and was merely an observation that studies involving multiple frequencies appeared to have less robust effects.
Ultimately Rogala and colleagues found that lack of specificity in the training approach seemed to elicit less clear and easily measurable results. However, they did not conclude that improved behavioral results were attributable to this narrower and easily measured focus, making their attempts to define the best approach somewhat inconclusive.
So, how do new practitioners learn the prevailing neurofeedback approaches? There are many perspectives in neurofeedback with sometimes widely varied methods, types of equipment, clinical populations, etc. In clinical practice, individuals providing neurofeedback training generally choose their training protocols based upon instructions from trainers and mentors, and therefore their approaches reflect these influences. In light of what may be considered a type of apprenticeship approach to the study of neurofeedback, which is a common entry point for new practitioners, it is worth discussing the various schools of neurofeedback training and how they have evolved and contributed to the development of the field of neurofeedback.
Glossary
ABA reversal design: a small N design where a baseline is followed by treatment and a return to baseline.
alpha blocking: the replacement of the alpha rhythm by low-amplitude desynchronized beta activity during movement, attention, mental effort like complex problem-solving, and visual processing.
alpha rhythm: 8-12-Hz activity that depends on the interaction between rhythmic burst firing by a subset of thalamocortical (TC) neurons linked by gap junctions and rhythmic inhibition by widely distributed reticular nucleus neurons. Researchers have correlated the alpha rhythm with relaxed wakefulness. Alpha is the dominant rhythm in adults and is located posteriorly. The alpha rhythm may be divided into alpha 1 (8-10 Hz) and alpha 2 (10-12 Hz).
alpha spindles: trains of alpha waves that are visible in the raw EEG and are observed during drowsiness, fatigue, and meditative practice.
amplitude: the strength of the EMG signal measured in microvolts or picowatts.
beta rhythm: 12-38-Hz activity associated with arousal and attention generated by brainstem mesencephalic reticular stimulation that depolarizes neurons in the thalamus and cortex. The beta rhythm can be divided into multiple ranges: beta 1 (12-15 Hz), beta 2 (15-18 Hz), beta 3 (18-25 Hz), and beta 4 (25-38 Hz).
beta spindles: trains of spindle-like waveforms with frequencies that can be lower than 20 Hz but more often fall between 22 and 25 Hz. They may signal ADHD, especially with tantrums, anxiety, autistic spectrum disorders (ASD), epilepsy, and insomnia.
bipolar (sequential) montage: a recording method that uses two active electrodes and a common reference.
common-mode rejection (CMR): the degree by which a differential amplifier boosts signal (differential gain) and artifact (common-mode gain).
delta rhythm: 0.05-3 Hz oscillations generated by thalamocortical neurons during stage 3 sleep.
desynchrony: pools of neurons fire independently due to stimulation of specific sensory pathways up to the midbrain and high-frequency stimulation of the reticular formation and nonspecific thalamic projection nuclei.
EEG activity: a single wave or series of waves.
epileptiform activity: spikes and sharp waves associated with seizure disorders.
Fast Fourier Transform (FFT): a mathematical transformation that converts a complex signal into component sine waves whose amplitude can be calculated.
frequency: the number of complete cycles that an AC signal completes in a second, usually expressed in hertz.
hertz (Hz): a unit of frequency measured in cycles per second.
infra-low-frequencies (ILF): frequencies below 0.1 Hz.
infra-slow-frequencies (ISF): frequencies below 0.1 Hz.
low resolution electromagnetic tomography (LORETA): Pascual-Marqui's (1994) mathematical inverse solution to identify the cortical sources of 19-electrode quantitative data acquired from the scalp.
posterior dominant rhythm (PDR): the highest-amplitude frequency detected at the posterior scalp when eyes are closed.
power: the amplitude squared and may be expressed as microvolts squared or picowatts/resistance.
protocol: a rigorously organized plan for training.
Quantitative EEG (qEEG): digitized statistical brain mapping using at least a 19-channel montage to measure EEG amplitude within specific frequency bins.
raw EEG signal: oscillating electrical potential differences detected from the scalp.
reference electrode: an electrode placed on the scalp, earlobe, or mastoid.
sampling rate: the number of times per second that an ADC samples the EEG signal.
sensorimotor rhythm (SMR): 13-15 Hz spindle-shaped sensorimotor rhythm (SMR) detected from the sensorimotor strip when individuals reduce attention to sensory input and reduce motor activity.
sleep spindles: waves that range from 12-15 Hz and last from 0.5 to several seconds widely distributed over the scalp and are observed during Stage 2 and 3 sleep.
standardized LORETA (sLORETA): a refinement of LORETA that estimates each voxel's electrical potentials without regard to their frequency, expresses normalized F-values, and achieves a 1-cubic-cm resolution.
surface Laplacian (SL) analysis: a family of mathematical algorithms that provide two-dimensional images of radial current flow from cortical dipoles to the scalp.
swLORETA: a more precise and accurate iteration of the LORETA source localization method.
theta/beta ratio (T/B ratio): the ratio between 4-7 Hz theta and 13-21 Hz beta, measured most typically along the midline and generally in the anterior midline near the 10-20 system location Fz.
theta rhythm: 4-8-Hz rhythms generated a cholinergic septohippocampal system that receives input from the ascending reticular formation and a noncholinergic system that originates in the entorhinal cortex, which corresponds to Brodmann areas 28 and 34 at the caudal region of the temporal lobe.
z-score training: neurofeedback protocol that reinforces in real-time closer approximations of client EEG values to those in a normative database.
References
Arns, M. W., Kleinnijenhuis, M., Fallahpour, K., & Breteler, M. H. . (2008). Golf performance enhancement by means of ‘real-life neurofeedback’ training based on personalized event-locked EEG profiles. Journal of Neurotherapy, 11(4), 11-18. https://doi.org/10.1080/10874200802149656
Ayers, M. E. (1977). EEG Neurofeedback to bring individuals out of Level Two Coma [Abstract]. Biofeedback and Self-Regulation, 20(3), 304–305.
Beauchamp, M. K., Harvey, R. H., & Beauchamp, P. H. (2012). An integrated biofeedback and psychological skills training program for Canada's Olympic short-track speedskating team. Journal of Clinical Sport Psychology, 6(1), 67–84. https://doi.org/10.1123/jcsp.6.1.67
Berger, H. (1929). Über das elektroenkephalogramm des menschen. Arch Psychiatr Nervenkr 87(1), 527–570.
Budzynski, T.H. (1973). Sonic applications of biofeedback produced twilight states. In D. Shapiro, & T. Barber et al. (Eds.). Biofeedback and self-control. Aldine Publishing Company.
Budzynski, T. H. (1977). Tuning in on the twilight zone. Psychology Today, 11, 38–44.
Budzynski, T. H. (1996). Brain brightening: Can neurofeedback improve cognitive process? Biofeedback, 24, 14–17.
Byers, A. P. (1998). Neurotherapy reference library (2nd ed.). Applied Psychophysiology and Biofeedback.
Campos da Paz, V. K., Garcia, A., Campos da Paz Neto, A., & Tomaz, C. (2018). SMR neurofeedback training facilitates working memory performance in healthy older adults: A behavioral and EEG study. Frontiers in Behavioral Neuroscience, 12, 321. https://doi.org/10.3389/fnbeh.2018.00321
Cheng, M. Y., Huang, C. J., Chang, Y. K., Koester, D., Schack, T., & Hung, T. M. (2015). Sensorimotor rhythm neurofeedback enhances golf putting performance. Journal of Sport & Exercise Psychology, 37(6), 626–636. https://doi.org/10.1123/jsep.2015-0166
Doppelmayr, M., & Weber, E. (2011). Effects of SMR and theta/beta neurofeedback on reaction times, spatial abilities, and creativity. Journal of Neurotherapy, 15(2), 115-129. https://doi.org/10.1080/10874208.2011.570689
Evans, J. R., Dellinger, M. B., & Russell, H. L. (Eds.) (2020). Neurofeedback: The first fifty years. Academic Press.
Fehmi, L. G., & Robbins, J. (2008). Sweet surrender-Discovering the benefits of synchronous alpha brainwaves (pp. 231-254). Measuring the immeasurable: The scientific case for spirituality. Sounds True, Inc.
Fein, G., Galin, D., Johnstone, J., Yingling, C. D., Marcus, M., & Kiersch, M. E. (1983).EEG power spectra in normal and dyslexic children. I. Reliability during passive conditions. Electroencephalogr Clin Neurophysiol, 55(4), 399-405. https://doi.org/10.1016/0013-4694(83)90127-x
Green, E. E., Green, A. M., & Walters, E. D. (1970). Voluntary control of internatial states: Psychological and physiological. Journal of Transpersonal Psychology, 9(1), 1–26.
Hardt, J. V., & Kamiya, J. (1978). Anxiety change through electroencephalographic alpha feedback seen only in high anxiety subjects. Science, 201(4350), 79-81. https://doi.org/10.1126/science.663641 PMID: 663641.
John, E. R., Ahn, H., & Prichep, L., Trepetin, M., Brown, D., & Kaye, H. (1980). Developmental equations for the electroencephalogram. Science, 210(4475), 1255-1258. https://doi.org/10.1126/science.7434026
Johnstone, J. & Gunkelman, J. (2003). Use of databases in QEEG evaluation. Journal of Neurotherapy, 7, 3-4, 31-52. https://doi.org/10.1300/J184v07n03_02
Kamiya, J. (1968). Conscious control of brain waves. Psychology Today, 1, 56-60.
Legarda, S. B., McMahon, D., Othmer, S., & Othmer S. (2011). Clinical neurofeedback: Case studies, proposed mechanism, and implications for pediatric neurology practice. J Child Neurol., 26(8),1045-1051. https://doi.org/10.1177/0883073811405052
Leong, S. L., Vanneste, S., Lim, J., Smith, M., Manning, P., & De Ridder, D. (2018). A randomised, double-blind, placebo-controlled parallel trial of closed-loop infraslow brain training in food addiction. Sci Rep 8(11659). https://doi.org/10.1038/s41598-018-30181-7
Lubar, J. F., & Bahler, W. W. (1976). Behavioral management of epileptic seizures following EEG biofeedback training of the sensorimotor rhythm. Biofeedback Self Regul, 1(1), 77-104. https://doi.org/10.1007/BF00998692. PMID: 825150.
Lubar, J. O., & Lubar, J. F. (1984). Electroencephalographic biofeedback of SMR and beta for treatment of attention deficit disorders in a clinical setting. Biofeedback and Self-Regulation, 9(1), 1–23. https://doi.org/10.1007/BF00998842
Lubar, J. F., Shabsin, H. S, Natelson, S. E., Holdson, S. E., Holder, G. S., Whittsett, S. F., Pamplin, W. E., & Krulikowski, D. I. (1981). EEG operant conditioning in intractable epileptics. Archives of Neurology, 38, 700–704.
Lubar, J. F., & Shouse, M. N. (1976). EEG and behavioral changes in a hyperkinetic child concurrent with training of the sensorimotor rhythm (SMR): A preliminary report. Biofeedback Self Regul,1(3), 293-306. https://doi.org/10.1007/BF01001170. PMID: 990355.
Marzbani, H., Marateb, H. R., & Mansourian, M. (2016). Neurofeedback: A comprehensive review on system design, methodology and clinical applications. Basic and clinical neuroscience, 7(2), 143–158.
Monastra, V. J., Lubar, J. F., Linden, M., VanDeusen, P., Green, G., Wing, W., Phillips, A., & Fenger, T. N. (1999). Assessing attention deficit hyperactivity disorder via quantitative electroencephalography: An initial validation study. Neuropsychology, 13(3), 424–433. https://doi.org/10.1037/0894-4105.13.3.424
Nuwer, M. R., & Coutin-Churchman, P. (2014). Brain mapping and quantitative electroencephalogram. Encyclopedia of the Neurological Sciences (2nd ed.). Elsevier.
Oken, B. S., & Chiappa, K. H. (1988). Short-term variability in EEG frequency analysis. Electroencephalogr Clin Neurophysiol, 69, 191–198. https://doi.org/10.1016/0013-4694(88)90128-9
Peniston, E. G., & Kulkosky, P. J. (1989). Alpha-theta brain wave training and beta-endorphin levels in alcoholics. Alcoholism, Clinical and Experimental Research, 13, 271 – 279. https://doi.org/10.1111/J.1530-0277.1989.TB00325.X
Peniston, E. G., & Kulkosky, P. J. (1990). Alcoholic personality and alpha-theta brain wave training. Medical Psychotherapy, 3, 37–55.
Peniston, E., Marrinan, D., Deming, W., & Kulkosky, P. (1993). EEG alpha-theta brainwave synchronization in Vietnam theatre veterans with combat-related post-traumatic stress disorder and alcohol abuse. Advances in Medical Psychotherapy, 6, 37-49.
Putman, J. A., Othmer, S. F., Othmer, S., & Pollock, V. E. (2005). TOVA results following inter-hemispheric bipolar EEG training. Journal of Neurotherapy: Investigations in Neuromodulation, Neurofeedback and Applied Neuroscience, 9(1), 37-52. https://doi.org/10.1300/J184v09n01_04
Raymond, J., Varney, C., Parkinson, L. A., & Gruzelier, J. H. (2005). The effects of alpha/theta neurofeedback on personality and mood. Brain research. Cognitive brain research, 23(2-3), 287–292. https://doi.org/10.1016/j.cogbrainres.2004.10.023
Ribas, V. R., Ribas, R., & Martins, H. (2016). The learning curve in neurofeedback of Peter Van Deusen: A review article. Dementia & Neuropsychologia, 10(2), 98–103. https://doi.org/10.1590/S1980-5764-2016DN1002005
Rogala, J., Jurewicz, K., Paluch, K., Kublik, E., Cetnarski, R., & Wróbel, A. (2016). The do's and don'ts of neurofeedback training: A review of the controlled studies using healthy adults. Frontiers in Human Neuroscience, 10, 301. https://doi.org/10.3389/fnhum.2016.00301
Scott, W. C., Kaiser, D., Othmer, S., & Sideroff, S. I. (2005). Effects of an EEG biofeedback protocol on a mixed substance abusing population. The American Journal of Drug and Alcohol Abuse, 31(3), 455–469. https://doi.org/10.1081/ada-200056807
Sherlin, L., Ford, L., Baker, A., & Troesch, J. (2015). Observational report of the effects of performance brain training in collegiate golfers. Biofeedback, 43, 64-72. https://doi.org/10.5298/1081-5937-43.2.06
Shouse, M. N., & Lubar, J. F. (1979). Operant conditioning of EEG rhythms and ritalin in the treatment of hyperkinesis. Biofeedback and Self-Regulation, 4, 299–312. https://doi.org/10.1007/BF00998960
Sittenfeld, P., Budzynski, T., & Stoyva, J. (1976). Differential shaping of EEG theta rhythms. Biofeedback and Self-Regulation, 1, 31–45. https://doi.org/10.1007/BF00998689
Smith, M. L., Collura, T. F., Ferrara, J., & de Vries, J. (2014). Infra-slow fluctuation training in clinical practice: A technical history. NeuroRegulation, 1(2), 187–207. https://doi.org/10.15540/nr.1.2.187
Smith, M. L., Leiderman, L., & de Vries, J. (2017). Infra-slow fluctuation (ISF) for autism spectrum disorders. In T. F. Collura & J. A. Frederick (Eds.), Handbook of clinical QEEG and neurotherapy. Routledge Taylor and Francis Group.
Sterman, M. B. (1976). Effects of brain surgery and EEG operant conditioning on seizure latency following monomethylhydrazine in the cat. Exp. Neurol., 50, 757-765. https://doi.org/10.1016/0014-4886(76)90041-8
Sterman M. B. (1996). Physiological origins and functional correlates of EEG rhythmic activities: implications for self-regulation. Biofeedback and Self-Regulation, 21(1), 3–33. https://doi.org/10.1007/BF02214147
Sterman, M. B., & Friar, L. (1972). Suppression of seizures in an epileptic following sensorimotor EEG feedback training. Electroencephalogr Clin Neurophysiol,33(1), 89-95. https://doi.org/10.1016/0013-4694(72)90028-4. PMID: 4113278
Sterman, M. B., Howe, R. C., & Macdonald, L. R. (1970). Facilitation of spindle-burst sleep by conditioning of electroencephalographic activity while awake. Science, 167(3921), 1146–1148. https://doi.org/10.1126/science.167.3921.1146
Sterman, M. B., Lopresti, R. W., & Fairchild, M. D. (1969). Electroencephalographic and behavioral studies of monomethylhydrazine toxicity in the cat. AMRL-TR-69-3, Aerospace Medical Research Laboratory, Air Force Systems Command, Wright-Patterson Air Force Base, Ohio.
Sterman, M. B., LoPresti, R. W., & Fairchild, M. D. (2010). Electroencephalographic and behavioral studies of monomethyl hydrazine toxicity in the cat. Journal of Neurotherapy, 14(4), 293-300, https://doi.org/10.1080/10874208.2010.523367
Strehl, U. (2014). What learning theories can teach us in designing neurofeedback treatments. Frontiers in Human Neuroscience, 8(894).
Swingle, P. (2014). Clinical versus normative databases: Case studies of clinical Q assessments. NeuroConnections.
Thatcher, R. W. (1998). Normative EEG databases and EEG biofeedback. Journal of Neurotherapy, 2(4), 8-39. https://doi.org/10.1300/J184v02n04_02
Thatcher, R. W., Biver, C. J., Soler, E. P., Lubar, J., & Koberda, J. L. (2020). New advances in electrical neuroimaging, brain networks and neurofeedback protocols. J. Neurol. Neurobiol., 6, 168. https://doi.org/10.16966/2379-7150.168
Thatcher, R. W., Lubar, J. F., & Koberda, J. L. (2019). Z-Score EEG biofeedback: Past, present, and future. Biofeedback, 47(4), 89–103. https://doi.org/10.5298/1081-5937-47.4.04
Thompson, L., & Thompson, M. (1998). Neurofeedback combined with training in metacognitive strategies: effectiveness in students with ADD. Applied Psychophysiology and Biofeedback, 23(4), 243–263. https://doi.org/10.1023/a:1022213731956
Thompson, T., Steffert, T., Ros, T., Leach, J., & Gruzelier, J. (2008). EEG applications for sport and performance. Methods, 45(4), 279–288. https://doi.org/10.1016/j.ymeth.2008.07.006
Wilson, V., Peper, E., & Moss, D. (2006) “The Mind Room” in Italian soccer training: The use of biofeedback and neurofeedback for optimum performance. Biofeedback, 34, 79-81.
D. INTRODUCTION TO ALPHA-THETA TRAINING
The EEG biofeedback (neurofeedback) approach known as alpha-theta (A-T) training is historically one of the first to be developed and is also one of the most widely used. This section will describe the evolution from early alpha training efforts to the current refinements and give an overview of the research and application of this approach. A demonstration of an A-T training session and various graphics will help readers grasp the concepts and outcomes of this type of training.

This section covers Underlying Theory, Cross-Frequency Synchronization, Local Field Potential, Global and Local Synchronization, History and Development, Benefits of A-T Training, Comparison of Interventions, A-T Training Protocols, Expected Results, Applications, Cautions (e.g., abreactions), Self-Medication, Substance/Behavioral Use Disorders, and Final Thoughts.
Underlying Theory -- Timing is Everything!
Oscillatory timing is the brain's fundamental organizer of neuronal information. According to Buzsaki (2006), brain activity is associated with multiple nested rhythms that emerge from integrated and coordinated activity mediated by small-world networks that interact with each other through hubs and nodes.
Cross-Frequency Synchronization
Slower frequencies organize and provide a matrix within which faster frequencies can function in a coordinated manner. Faster frequencies emerge from “bound networks” (Gunkelman, 2005), meaning that as networks responsible for slower, rhythmic frequencies become synchronized or bound together, faster frequencies emerge due to neuronal activity arising from these bound networks. Scalp EEG frequencies represent the synchronized firing of multiple neurons. The more neurons firing synchronously at a given frequency, the higher the voltage (amplitude) of that frequency.
For example, when the eyes are open, visual processing systems made up of millions of neurons are busy responding to incoming sensory input from the thalamocortical relay (TCR) system that involves ascending pathways from the thalamus mediated by the reticular nucleus of the thalamus (nRt). When the eyes are closed and visual input is no longer present, the visual processing neurons respond to a rhythmic signal, coming from the TCR system that results from interactions between the thalamus and the nRt, which also respond to ascending neurochemical inputs from the brainstem and reticular activating system (Cox et al., 1997).
This rhythm is commonly called the alpha rhythm (Berger, 1929) or the posterior dominant rhythm (PDR). When visual neurons collectively fire in response to this rhythmic input from the thalamus, the amplitude of the alpha signal increase.
This is called the alpha response.
Visual neurons begin to process the incoming information relayed by the TCR system when the eyes are opened. So fewer neurons are responding to the rhythmic PDR input, and the amplitude of alpha measured from the scalp decreases.
This is known as alpha blocking.
The same number of neurons may still be active, but since they are now functioning more independently and each grouping of neurons has a separate task, the overall voltage of the EEG as a whole decreases. Reduced synchrony of neuronal firing results in decreased amplitude.
Benefits of A-T Training
Research participants reported "transformative" experiences, including:
Spiritual and religious imagery
Memory recall experiences
Insight into personal behaviors and patterns
Changes in interpersonal relationships
Understanding of purpose
Resolution of long-standing anxiety and depression
Release of trauma responses and reactivity
Improved self-esteem /self-image
Abstinence from substance and behavioral addictions
The auditory feedback to the trainee is that of reward when theta power increases over alpha. This is called a “crossover” since it is a transition from the common baseline state of dominant alpha to that of theta. The sensation for the trainee is a sense of floating, or twilight state felt just before falling asleep. It is also an EEG pattern commonly produced with hypnosis and deep relaxation. [34-38]. The relaxation state is enhanced when the trainee can increase and maintain for several minutes theta power over alpha power by 1mV and maintain reduced beta. Sensor placement at parietal and occipital locations is used, and training is with eyes closed. The procedure more often produces visual memories (Johnson, 2013).
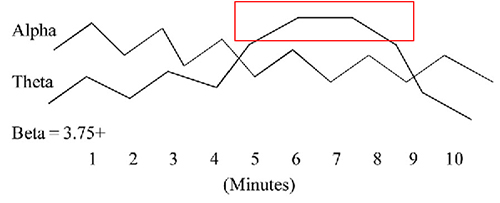
One of the performance benefits of synchronized theta activity (4-7 Hz) is encoding new information or working memory. It is also applied as a cognitive training routine in pre-performance preparations using imagery (e.g., dance performance; Gruzelier, 2014). Military applications might include freefall training, movement to contact, and equipment assembly, to name a few. Whether in performing arts, professional sports, or even among business executives, the neurofeedback application of aiding a calm and memory-enhanced brain state has grown in use among sports psychologists (Harmison, 2006). Increased theta activity is highlighted by the red box shown below.
Comparison of Interventions
Patricia Norris, Steven Fahrion, and others split off from the Menninger Foundation in 1993 to form the Life Sciences Institute of Mind-Body Health. They conducted a 5-year study of A-T training in a prison population (described in Norris, 2017).
Two groups of participants all received the following self-regulation, educational and supportive components of the study: Temperature training, breath training, didactic presentations on philosophy, psychodynamic aspects of self, and psychosynthesis exercises. Participants were treated with “deference, respect, and compassion.” One group also received 45 daily sessions of A-T neurofeedback training.
The results of a 2-year follow-up of participants (N = 35) showed encouraging results compared to standard substance use disorder (SUD) treatment in prison environments, typically with 10-20% "survival" rates defined by abstinence, no violations, and no criminal activity.
At a 1-year follow-up, 78% of the A-T group survived compared with 75% of controls. At a 2-year follow-up, 69% of the A-T group survived compared with 64% of the control group.
Younger participants had somewhat different results, suggesting that the A-T training was a more critical component for this group. At a 1-year follow-up, the A-T group had a 74% survival rate compared to 54% for controls. At a 2-year follow-up, the A-T group had a 60% survival rate compared with 45% for controls.
African-American and Hispanic participants also had results suggesting improved results with A-T training. At a 1-year follow-up, the A-T group had a 70% survival rate compared to 48% for controls. At a 2-year follow-up, the A-T group had a 48% survival rate compared with 40% for controls.
What Was the Mechanism of Change?
This study shows that the overall treatment program was quite effective compared to standard interventions. The difference between A-T and control group results was not statistically significant when the groups were viewed as a whole. However, age and ethnicity functioned as moderator variables.The overall design and implementation of the program was likely the most crucial aspect, and the A-T training was one effective component of that broader effort.
The relationship between the trainer and the client is one of the most important components of any intervention.
This is particularly critical when providing A-T training because the desirable state identified as the crossover state requires complete trust on the part of the client so that they can let go of emotional, psychological, and physical defenses that would prevent them from allowing the type of disconnection from outside sensory input necessary for the state to occur.
Trainers must work to engender this trusting relationship before implementing A-T training. This is one reason why many trainers use a variety of additional interventions before and/or concurrently with A-T training.
Some of those interventions include:
Breathing, including heart rate variability (HRV) training
Guided relaxation
Peripheral temperature training
EMG biofeedback
Electrodermal biofeedback
SMR, beta, and other amplitude-based neurofeedback protocols
Z-score based neurofeedback approaches
Hypnotic inductions
Behavior change scripts and suggestions
Post-session psychotherapy, processing, and interpretation
What Role Does A-T Training Play in the Changes Experienced By Clients?
The client’s ability to enter the desired state may depend upon many factors: set and setting, expectations, trainer self-training, and interpersonal skills.The ability to enter the reverie state appears to result in significant opportunities for change, which can also occur from multiple interventions such as:
Mindfulness meditation
Biofeedback, including HRV training
A-T training
Other types of EEG biofeedback
Other self-help and behavior change interventions such as hypnosis and psychotherapy
Interventions that lead to desirable states appear to have one thing in common – it is difficult for the client or student to know when they are in the state, getting close to the state, or very far away from the state.
A-T neurofeedback, when done correctly, provides constant information about where one is on the continuum. This allows the client to move toward and experience the desired state more quickly and facilitates faster and more accurate skill acquisition.
A-T Training Protocols
A variety of approaches to A-T training have been developed. The initial protocol developed by Eugene Peniston (Peniston, 1989, 1990) involved two separate audio tones: alpha amplitude and the other representing theta amplitude. When alpha amplitude increased, the alpha tone occurred more frequently, and when theta amplitude increased, the theta tone occurred more often. In this way, the trainee could perceive the relative amount (amplitude or voltage) of each frequency band and could practice various strategies to accomplish this task to increase theta and decrease alpha. The feedback guided the trainee and reinforced the desired state change behavior, resulting in the so-called crossover state.
Some trainers have found this protocol somewhat complicated to administer. Peniston prescribed threshold changes to elicit a shift to the crossover state, beginning with a setting that rewarded alpha 50 to 70% of the time and rewarded theta approximately 20 to 40% of the time. As training progressed, the percentage of the alpha reward tone was allowed to decrease as the signal amplitude decreased. In contrast, the theta reward tone was allowed to increase if the theta amplitude increased. However, the instruction was to maintain the theta reward tone at approximately 40%, even if the voltage decreased, to provide at least that minimal level of positive reinforcement.
Some practitioners attempting to train clients with this approach found this difficult to administer correctly, and some clients became confused about the desired signal. Subsequently, a simplified and widely-used approach was developed that utilizes the theta-alpha ratio to simplify the training protocol and provide a single-tone feedback signal representing the crossover state. Often the trainee is provided with a tone or some other audio indicator that they have exceeded a certain threshold indicating that theta has increased in amplitude over alpha amplitude, suggesting that the crossover state has occurred.
This is a simple protocol that has been incorporated into a variety of commercial neurofeedback platforms developed by equipment and software manufacturers. Concerns about this simplified approach include the lack of specificity in the training protocol and the lack of feedback to inhibit undesirable changes in the EEG, leading to negative reactions and the lack of a well-defined goal.
The theta/alpha ratio is not precise since the band frequencies are quite broad. An increase in the slower theta component can increase the feedback reward signal but may not represent the desirable crossover state. Additionally, the client may not even produce typical alpha increases initially. Therefore, the simple ratio feedback approach lacks the precision to identify desirable states and differentiate between causal factors resulting in increases or decreases in positive feedback.
Finally, there is no indicator of whether the client is close to the goal, far away from the goal or somewhere in between. A single tone representing the crossover leaves the client wondering where they are on the continuum of alertness and awareness.
Targeted Training - 6-9 Hz
Following significant experimentation and clinical experience, John Anderson and others developed a protocol that involves training the frequency band from 6-9 Hz, which is the actual frequency band of the so-called crossover state. This frequency band straddles the standard 4-8 Hz theta and 8-12 Hz alpha frequency bands. This protocol is combined with an inhibit channel that prevents an increase in 2-6 Hz activity. When allowed to increase during A-T training, it results in an abreaction or negative reaction, sometimes involving traumatic recall or depersonalization experiences.Through experience, using the 2-6 Hz frequency band to inhibit this transition to a more sleep-like state appears to prevent these negative reactions. Additionally, a frequency band from approximately 13-36 Hz is used to prevent an increase in faster frequency EEG patterns associated with cognitive activity, thereby ensuring that the client remains in the relaxed reverie state without engaging in thinking and problem-solving activities. This does represent a somewhat more complex approach to the A-T training protocol but is more likely to produce a desirable outcome without the dangers of negative reactions or excessive cognitive activity.
Reward Feedback
Reward feedback is a proportional audio signal, usually music, that increases and decreases in volume in direct proportion to the changes in 6-9 Hz amplitude. Clients can select their audio from calming, relaxing musical choices.The volume changes in the audio selection allow the client to know continuously where they are on the continuum from a typical eyes-closed alert state to the deep reverie state associated with the optimum experience. This is similar to the hot/cold game played by children.
Inhibit Feedback
The low-frequency inhibit (2-6 Hz) feedback is a recording of birds chirping that only sounds above a set threshold and becomes louder as the amplitude of this signal increases. This allows the client to be gently alerted when shifting into this lower frequency state without being startled out of the desirable reverie state. Most clients associate birds chirping with waking up in the morning, so it seems to represent an intuitive indicator that is easy to remember.The high-frequency inhibit (10-36 Hz) is a recording of ocean wave sounds with an inverse proportional relationship to the amplitude of this signal. It becomes louder as the "busy brain" decreases, thus rewarding a decrease in cognitive activity. The ocean wave sound and the rewarding music blend to create a calming and reinforcing auditory environment that encourages the correct state while providing constant, meaningful feedback to guide the client.
Sample Training Session
The image below shows the beginning of a 2-channel A-T training session. Note that the eyes closed EEG shows no clear posterior rhythm (alpha) and that peripheral skin temperature is 79.94o F measured from the small finger of the non-dominant hand, indicating increased SNS activity. GSR/SC is shown as resistance in Kohms, where higher values indicate decreased sweat gland activity measured from the palm of the non-dominant hand. Current GSR readings are 68.04 Kohms, which indicates increased arousal/anxiety. The sum of the P3 + P4 6-9 Hz activity is 9.75 μV, while 8-12 Hz alpha is 10.62 μV. Graphic © John S. Anderson.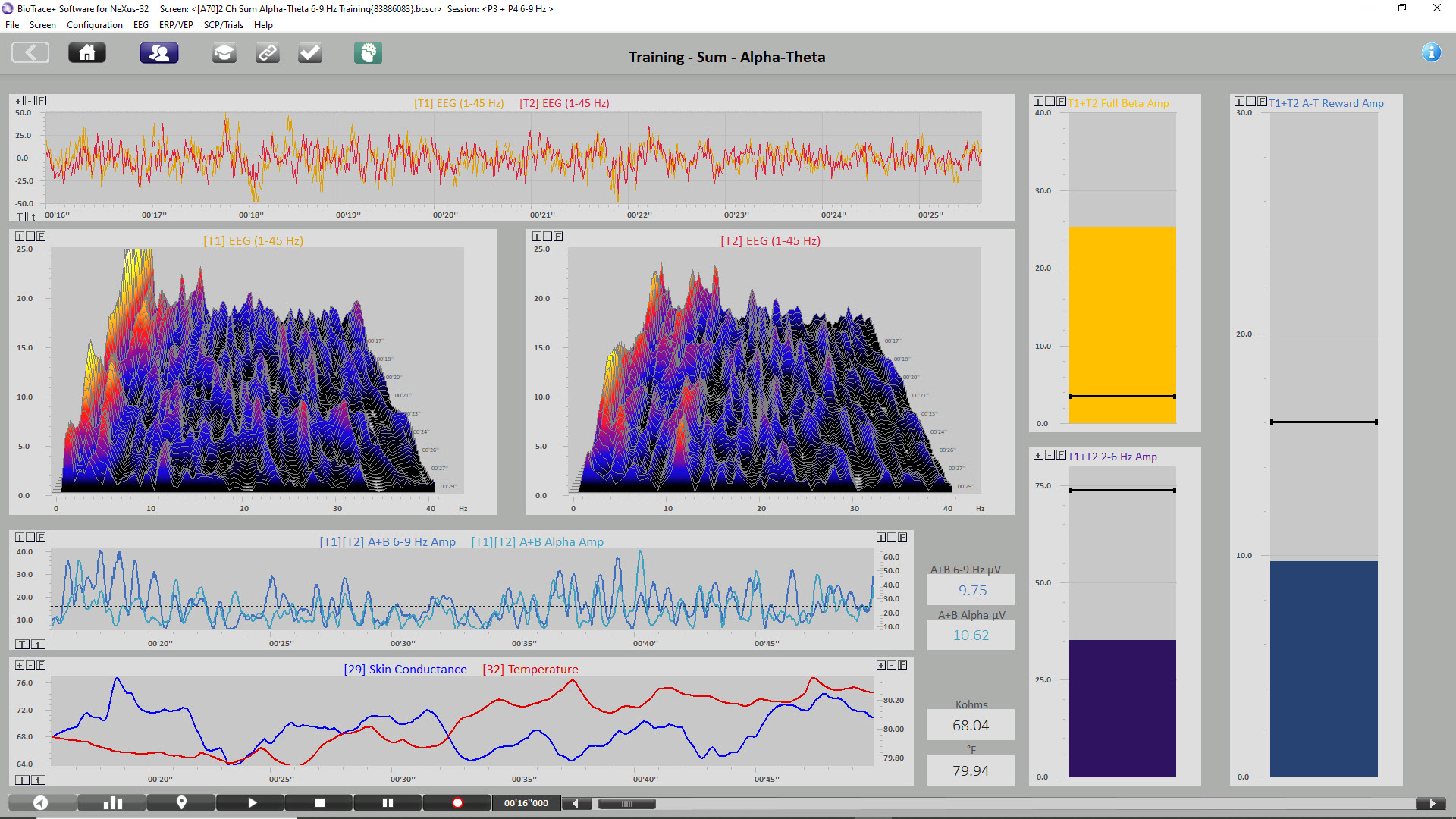
The image below shows the same session when the initial increase in alpha amplitude occurs. Peripheral skin temp has increased to 80.50o F, and GSR has increased to 121.12 Kohms, indicating decreased arousal. P3 + P4 6-9 Hz activity has increased to 14.34 μV, while 8-12 Hz alpha has increased to 24.26 μV. Graphic © John S. Anderson.
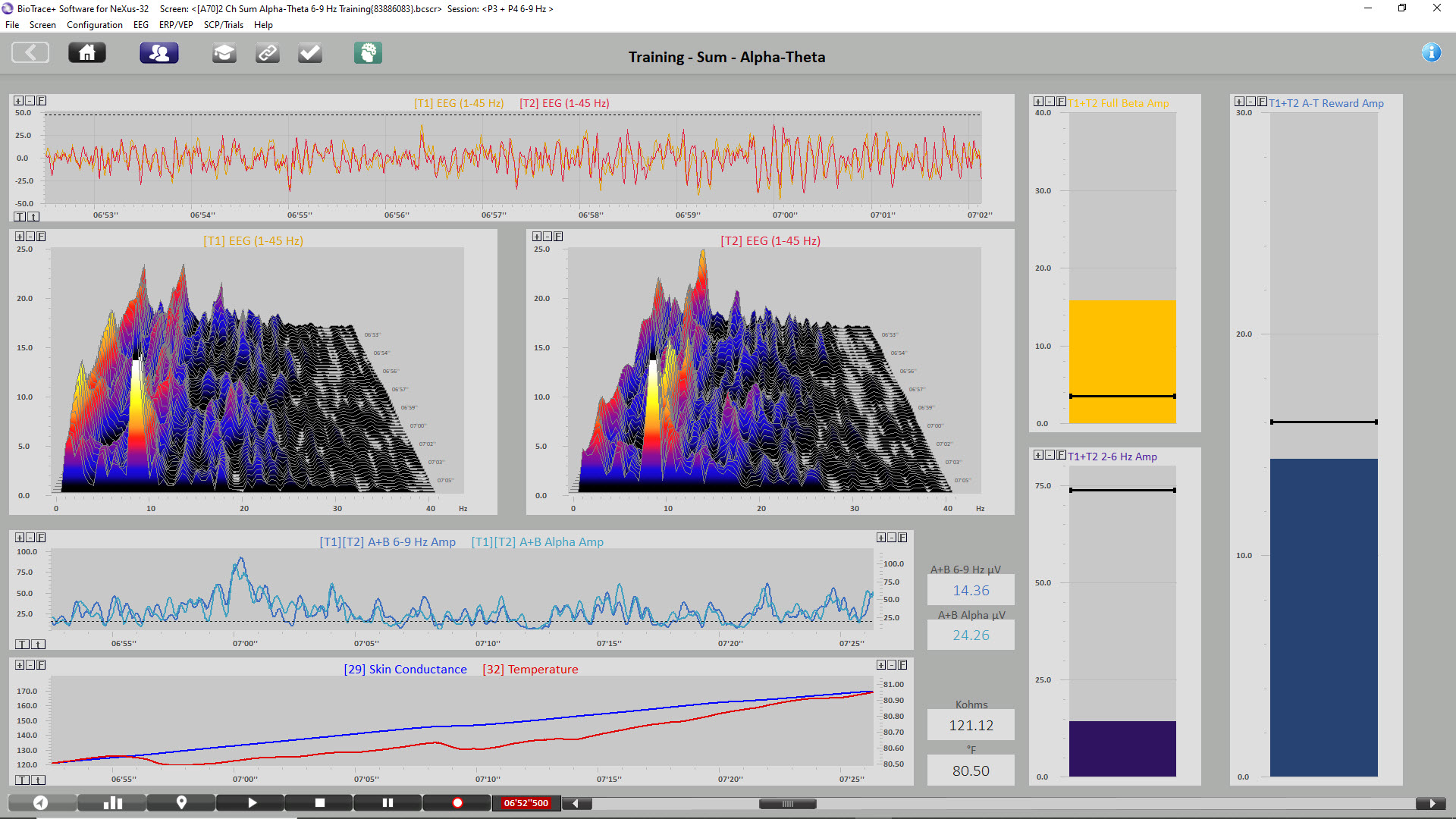
The image below shows the same session near the end. Peripheral skin temp has increased to 90.57
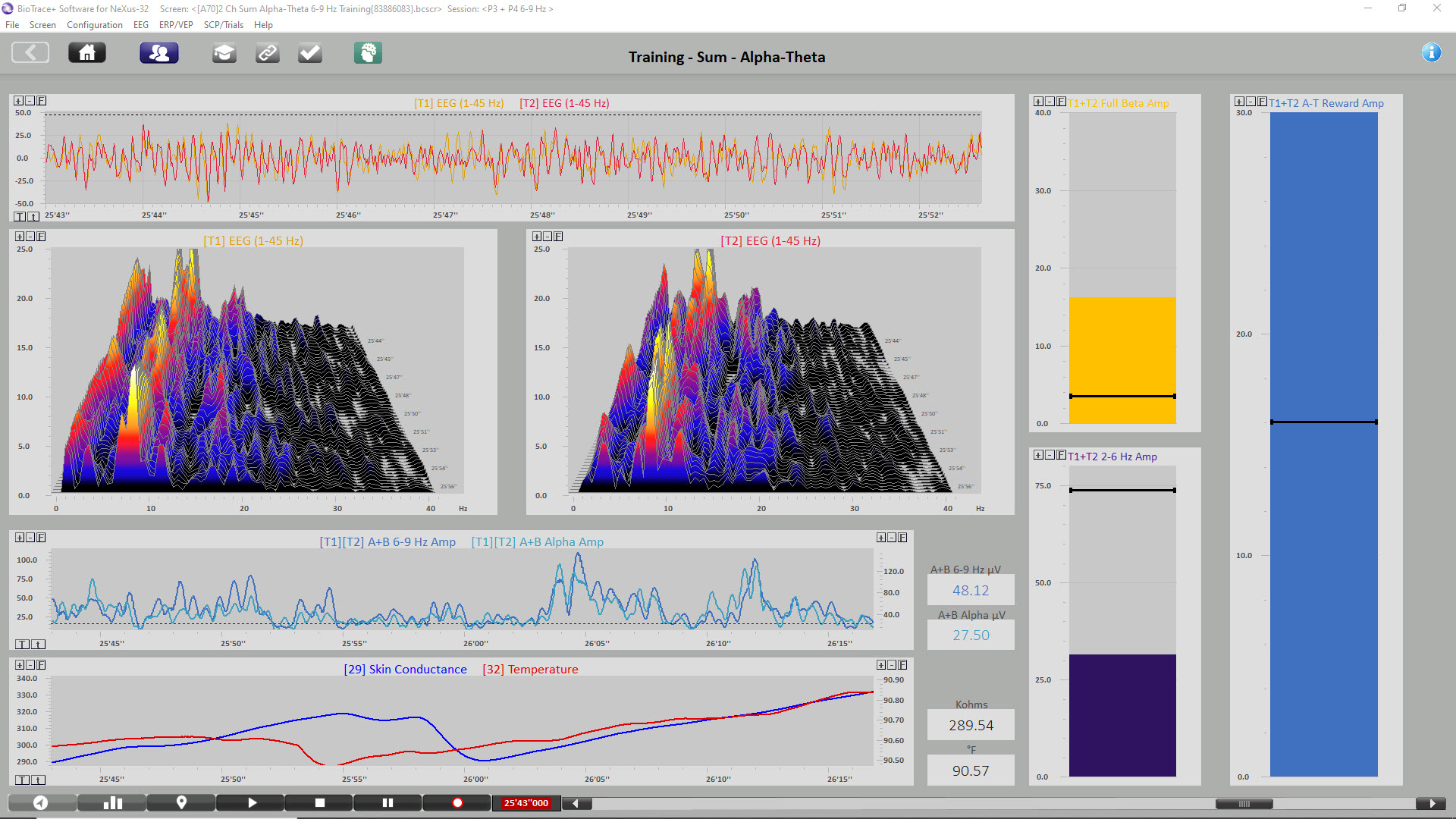
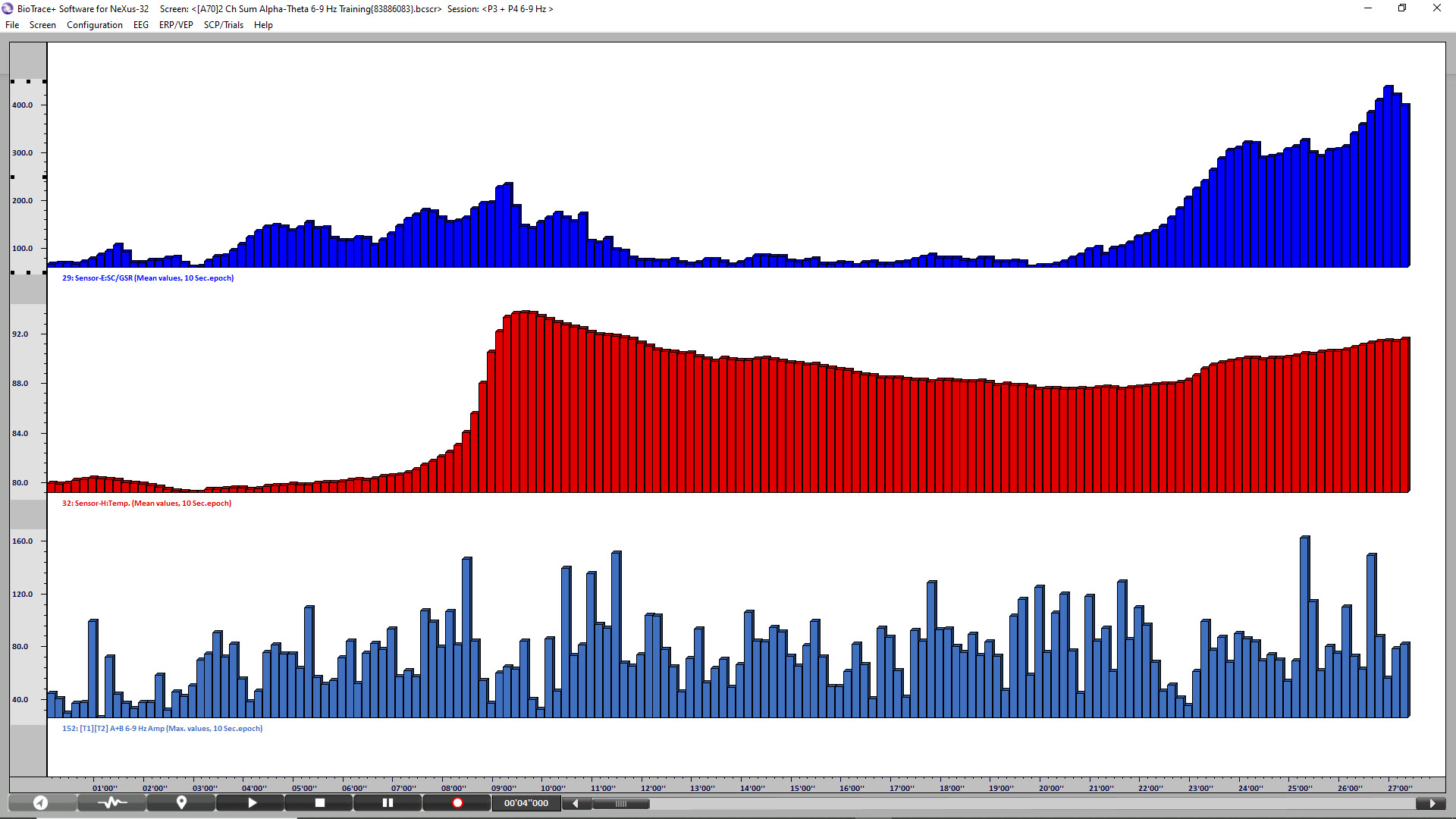
The final image is a summary screen that shows an increase in GSR (top), temperature (middle), and P3 + P4 6-9 Hz activity (bottom) across the session. Graphic © John S. Anderson.
Expected Results
This state encouraged by this training appears to be similar to that experienced during mindfulness meditation practice. The participant or trainee experiences what is sometimes called a witness state, or the observation of the flow of consciousness, memory, and internal thoughts, without actively pursuing these thoughts. This training approach operationalizes this training so that the client experiences indicators via audio rewards that encourage remaining in the state and discourage movement in either direction towards a deeper, more sleep-like state or an activated cognitive state.
Individuals participating in this training report increased receptiveness to suggestion, the experience of a reverie state, free association and/or consciousness streaming experiences, experiences of representational and/or symbolic/spiritual/religious imagery, and experiences of emotional resolution, insight, and understanding.
The theories associated with this approach suggest that this state of brain activity is similar to that experienced in hypnotic induction states and in deeply relaxed and light sleep states associated with memory consolidation.
Applications
Whatever the approach, A-T training has been utilized for a variety of different interventions, mostly falling into the loose categories of stress-related conditions (Nicholson et al., 2020; Peniston, 1989, 1990), addictive behavior (Burkett, 2005; Kaiser, 2005; Peniston, 1989, 1990) and optimal performance (Gruzelier, 2014).
An example of optimum performance training using A-T training can be seen in Gruzelier’s (2014) work with musicians in a study published in Biological Psychology. He demonstrated improvements in three music domains: creativity/musicality, technique, and communication/presentation. His study showed improvements in all blinded, independently rated scales for the alpha-theta training group compared to an additional EEG training group using an SMR (12-15 Hz) reward and a non-training control group.

Caption: John Gruzelier
These results demonstrated that the effect is specific to the type of training (i.e., the frequency band or reward) as the SMR training group while improving technical scores associated with attention and motor control, did not show the same improvement in the other scales. The study also showed improved ratings overall for A-T training compared to a control condition.
Cautions
Some neurofeedback providers (Thompson & Thompson, 2003) suggest A-T training be used in the context of clinical support when an individual is suspected to have experienced trauma or a trauma-related condition. Therefore, providers utilizing this intervention should have advanced training beyond introductory-level course work.

Caption: Michael and Lynda Thompson
As we have noted, much of this concern results from a lack of understanding of the process associated with this protocol and an incorrect application of the training, including a lack of appropriate indicators to the client regarding undesirable frequency activity.
Client preparation is essential, as is true with any intervention. Clients should be instructed to immediately inform the neurofeedback provider of any negative reactions so that corrective training and/or a calming intervention such as paced breath training via heart rate variability feedback can be implemented.
One of the characteristics of this training is a decrease in limbic system arousal. This is seen in decreased anxiety, easier transitions to sleep and deeper, more restful sleep, a long-term overall reduction of limbic system activation, insulation from the stress of daily life events, and improved state management and stability.
There is evidence that this state may also be similar to that identified in fMRI studies as the default mode network, sometimes known as the resting state network. This network of connections in the brain is associated with what is sometimes known as the self-referential state that accompanies a state of self-awareness associated with memory recall and non-directed cognition that appears strikingly similar to that described by individuals participating in A-T training.
The technical aspects of this type of training are specific to the training platform, and practitioners will need to familiarize themselves with their systems' specific software and hardware characteristics.
Typical indications for A-T training include clients with the following presenting symptoms.
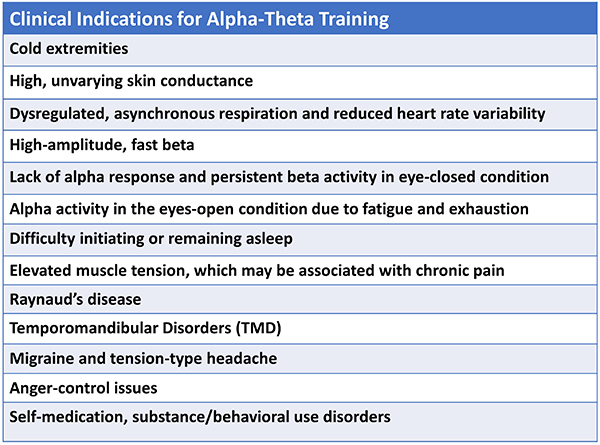
Practitioners have used various pre-training options to facilitate their client’s ability to access the desirable state during A-T training. Peniston (1989, 1990) initially used 10 sessions of hand temperature training to criteria, which was 96°F. Scott and Kaiser (2005) used beta and SMR training to stabilize the nervous system before continuing A-T training. Other practitioners use heart rate variability training and/or a combination of training approaches depending on the client’s needs. Some optimal performance practitioners begin directly with A-T training, which may be their sole neurofeedback intervention.
Training protocols vary depending on the environment, the clientele, and the ability of the practitioner to provide sessions. Initially, Peniston used training sessions once per day for 30 sessions. Scott and Kaiser also used daily training sessions for 30 A-T training sessions. Other practitioners have used various approaches, including once or twice weekly sessions and some have utilized twice daily intensive training programs.
Peniston's (1989, 1990) initial approach used the O1 electrode location based on the 10-20 international electrode placement system. Subsequent practitioners have also utilized the Pz electrode location or the P3 and P4 electrode locations to facilitate two-channel A-T training.
Peniston’s approach to training included behavior change scripts that would be read to the participant before the training session. He also conducted post-session debriefing conversations with his participants (personal communication, 1995). Other practitioners have also used behavior change scripts or scenarios, affirmations, behavior change, ideal behavior/life scenario instructions, and tape-recorded relaxation practices before each A-T session. The following show initial A-T training sessions and subsequent training sessions, indicating the crossover event occurring in single-channel and two-channel training approaches.
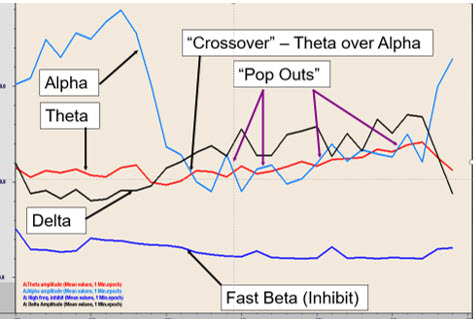
Initial A-T neurofeedback training session.The initial increase in alpha amplitude is followed by a decrease in alpha amplitude to the point where the theta amplitude is greater than the alpha amplitude resulting in brief crossover events.
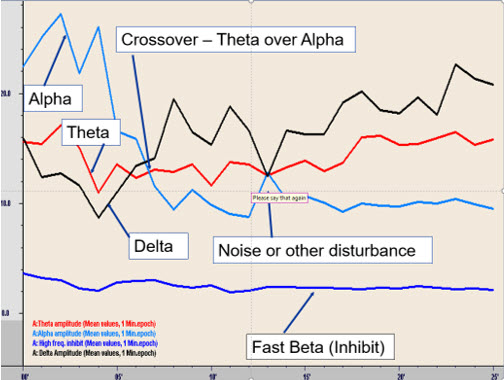
A later session of A-T training initially indicated the increase in alpha as the eyes are closed, followed by a transition to a decreased alpha with increased theta amplitude, resulting in a prolonged crossover experience.
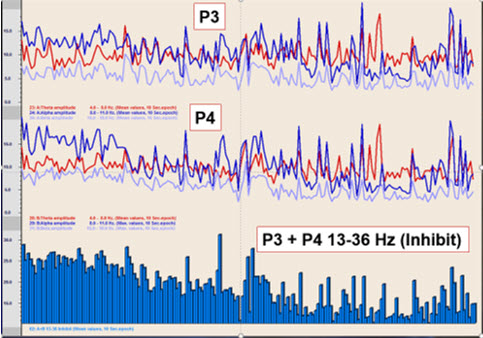
In an early training session with the client using two channels at parietal locations P3 and P4, there are some crossover events. The primary finding is a significant decrease in the 13-36 Hz activity that indicates a marked decrease in the initial overactivity of the central nervous system.
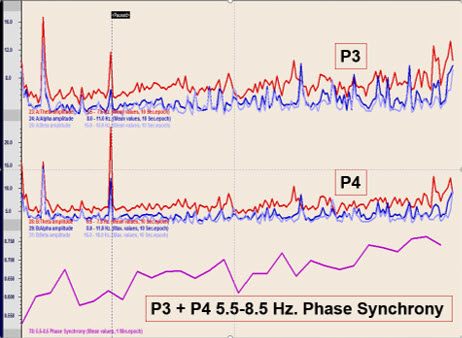
This graphic represents a later session with the same client showing prolonged crossover and an increase in phase synchrony between the two parietal locations in the 5.5-8.5 Hz frequency band used in this case.
This video takes the viewer through an A-T training demonstration using the training approach developed by John Anderson. The demonstration uses saved session data to illustrate the functions and discuss the threshold settings and training concepts. The video begins with a black screen, and the visual portion doesn't begin until the 1-minute mark.
Final Thoughts
Clients often have presenting conditions requiring other training approaches, specifically for ADHD and other attention issues, cognitive processing/slow processing issues, memory issues, sensory processing issues, and more. A-T training is often introduced following successful resolution of these issues. A-T training may help clients integrate and consolidate the changes that have occurred and may provide "perspective" on these changes and how they will affect their lives.
All biofeedback aims to help the client learn skills that they can then apply for the rest of their life. As trainers, we want our clients to become independent, self-regulating individuals with new choices and opportunities. We want our clients to function optimally, no matter where they started. We want to help reduce limitations and restrictions, so clients are free to pursue whatever goals they wish.
Glossary
alpha blocking: arousal and specific forms of cognitive activity may reduce alpha amplitude or eliminate it entirely while increasing EEG power in the beta range.
alpha response: an increased alpha amplitude.
alpha rhythm: 8-12-Hz activity that depends on the interaction between rhythmic burst firing by a subset of thalamocortical (TC) neurons linked by gap junctions and rhythmic inhibition by widely distributed reticular nucleus neurons. Researchers have correlated the alpha rhythm with relaxed wakefulness. Alpha is the dominant rhythm in adults and is located posteriorly. The alpha rhythm may be divided into alpha 1 (8-10 Hz) and alpha 2 (10-12 Hz).
alpha spindles: trains of alpha waves that are visible in the raw EEG and are observed during drowsiness, fatigue, and meditative practice.
alpha-theta training: a protocol to slow the EEG to the 6-9 Hz crossover region while maintaining alertness.
amplitude: the strength of the EMG signal measured in microvolts or picowatts.
beta rhythm: 12-38-Hz activity associated with arousal and attention generated by brainstem mesencephalic reticular stimulation that depolarizes neurons in the thalamus and cortex. The beta rhythm can be divided into multiple ranges: beta 1 (12-15 Hz), beta 2 (15-18 Hz), beta 3 (18-25 Hz), and beta 4 (25-38 Hz).
beta spindles: trains of spindle-like waveforms with frequencies that can be lower than 20 Hz but more often fall between 22 and 25 Hz. They may signal ADHD, especially with tantrums, anxiety, autistic spectrum disorders (ASD), epilepsy, and insomnia.
bipolar (sequential) montage: a recording method that uses two active electrodes and a common reference.
common-mode rejection (CMR): the degree by which a differential amplifier boosts signal (differential gain) and artifact (common-mode gain).
crossover state: the 6-9 Hz band targeted by A-T training.
default mode network (DMN): frontal, temporal, and parietal lobe circuits that are active during introspection and daydreaming and relatively inactive when pursuing external goals.
delta rhythm: 0.05-3 Hz oscillations generated by thalamocortical neurons during stage 3 sleep.
EEG activity: a single wave or series of waves.
epileptiform activity: spikes and sharp waves associated with seizure disorders.
Fast Fourier Transform (FFT): mathematical transformation that converts a complex signal into component sine waves whose amplitude can be calculated.
frequency: the number of complete cycles that an AC signal completes in a second, usually expressed in hertz.
functional magnetic resonance imaging (fMRI): an imaging technique that indirectly detects brain regions' oxygen use during specific tasks.
hertz (Hz): the unit of frequency measured in cycles per second
posterior dominant rhythm (PDR): the highest-amplitude frequency detected at the posterior scalp when eyes are closed.
power: amplitude squared and may be expressed as microvolts squared or picowatts/resistance.
Quantitative EEG (qEEG): digitized statistical brain mapping using at least a 19-channel montage to measure EEG amplitude within specific frequency bins.
sensorimotor rhythm (SMR): 13-15-Hz spindle-shaped sensorimotor rhythm (SMR) detected from the sensorimotor strip when individuals reduce attention to sensory input and reduce motor activity.
theta/beta ratio (T/B ratio): the ratio between 4-7 Hz theta and 13-21 Hz beta, measured most typically along the midline and generally in the anterior midline near the 10-20 system location Fz.
theta rhythm: 4-8-Hz rhythms generated a cholinergic septohippocampal system that receives input from the ascending reticular formation and a noncholinergic system that originates in the entorhinal cortex, which corresponds to Brodmann areas 28 and 34 at the caudal region of the temporal lobe.
z-score training: a neurofeedback protocol that reinforces in real-time closer approximations of client EEG values to those in a normative database.
References
Ancoli, S., & Kamiya, J. (1978). Methodological issues in alpha biofeedback training. Biofeedback and Self-Regulation, 3(2). 159-183. https://doi.org/10.1007/bf00998900
Berger, H. (1929). Über das Elektrenkephalogramm des Menschen. Archiv f. Psychiatrie, 87, 527–570.
Boyce, R., Glasgow, S. D., Williams, S., & Adamantidis, A. (2016). Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science, 352(6287), 812-816. https://doi.org/10.1126/science.aad5252
Brown, B. (1974). New mind, New body: Biofeedback: New directions for the mind. Harper & Row.
Budzynski, T. H. (1976). Biofeedback and the twilight states of consciousness. In G. E. Schwartz & D. Shapiro (Eds.). Consciousness and self-regulation. Springer.
Burkett, V. S., Cummins, J. M., Dickson, R. M., & Skolnick, M. (2005). An open clinical trial utilizing real-time EEG operant conditioning as an adjunctive therapy in the treament of crack cocaine dependence. Journal of Neurotherapy, 9(2). https://doi.org/10.1300/J184v09n02_03
Buzsáki, G. (2006). Rhythms of the brain. Oxford University Press.
Cox, C. L., Huguenard, J. R., & Prince, D. A. (1997). Nucleus reticularis neurons mediate diverse inhibitory effects in thalamus. Proceedings of the National Academy of Sciences, 94(16), 8854-8859. https://doi.org/10.1073/pnas.94.16.8854
Davey, C. G., & Harrison, B. J. (2018). The brain's center of gravity: how the default mode network helps us to understand the self. World Psychiatry: Official Journal of the World Psychiatric Association (WPA), 17(3), 278-279. https://doi.org/10.1002/wps.20553
Green, E., & Green, A. (1977). Beyond biofeedback. Knoll Publishing Company.
Gruzelier, J. H., Holmes, P., Hirst, L., Bulpin, K., Rahman, S., van Run, C., & Leach, J. (2014). Replication of elite music performance enhancement following alpha/theta neurofeedback and application to novice performance and improvisation with SMR benefits. Biological Psychology, 95, 86-107. https://doi.org/10.1016/j.biopsycho.2013.11.001
Hardt, J., & Kamiya, J. (1976). Some comments on Plotkin’s self-regulation of the electroencephalographic alpha. J. Exp. Psychol., 105(1), 100-108. https://doi.org/10.1037/0096-3445.105.1.100
Harmison, R. J. (2006). Peak performance in sport: identifying ideal performance states and developing athletes’ psychological skills. Prof. Psychol. Res. Pract. 37, 233–243. https://doi.org10.1037/0735-7028.37.3.233
Johnson, M. L., Bodenhamer-Davis, E., Bailey, L. J., & Gates, M. S. (2013). Spectral dynamics and therapeutic implications of the theta/alpha crossover in alpha-theta neurofeedback. J of Neurotherapy, 17(1), 3-34. https://doi.org/10.1080/10874208.2013.758968
Kamiya, J. (1961). Behavioral, subjective, and physiological aspects of drowsiness and sleep. In D. W. Fiske, & S. R. Maddi (Eds.). Functions of varied experience. Dorsey Press.
Kamiya, J. (1962). Conditioned discrimination of the EEG alpha rhythm in Humans. Abstract presented at the Western Psychological Association meeting.
Kamiya, J. (1968). Conscious control of brain waves. Psychology Today, 1, 56-63.
Kamiya, J. (1969). Operant control of the EEG alpha rhythm and some of its reported effects on consciousness. In C. Tart (Ed.), Altered states of consciousness. John Wiley and Sons.
Nicholson, A. A., Densmore, M., MicKinnon, M. C., Neufeld, R. W. J., Frewen, P. A. Théberge, J., Jetly, R., Richardson, J. D., & Lanius, R. A. (2019). Machine learning multivariate pattern analysis predicts classification of posttraumatic stress disorder and its dissociative subtype: A multimodal neuroimaging approach. Psychol Med, 49(12), 2049-2059. https://doi.org/10.1017/S003329171800286
Nicholson, A. A., Ros, T., Densmore, M., Frewen, P. A., Neufeld, R. W. J., Théberge, J., Jetly, R., & Lanius, R. A. (2020). A randomized, controlled trial of alpha-rhythm EEG neurofeedback in posttraumatic stress disorder: A preliminary investigation showing evidence of decreased PTSD symptoms and restored default mode and salience network connectivity using fMRI. Neuroimage Clin. 28, 102490. https://doi.org/10.1016/j.nicl.2020.102490
Nicholson, A. A., Ros, T., Jetly, R., & Lanius, R. (2020). Regulating posttraumatic stress disorder symptoms with neurofeedback: Regaining control of the mind. Journal of Military Veteran and Family Health 6(S1), 3-15. https://doi.org/10.3138/jmvfh.2019-0032
Peniston, E. G., & Kulkosky, P. J. (1989). Alpha-theta brain wave training and beta-endorphin levels in alcoholics. Alcoholism, Clinical and Experimental Research, 13, 271–279. https://doi.org/10.1111/j.1530-0277.1989.tb00325.x
Peniston, E. G., & Kulkosky, P. J. (1990). Alcoholic personality and alpha-theta brain wave training. Medical Psychotherapy, 3, 37–55.
Scott, W. C., Kaiser, D., Othmer, S., Sideroff, S. I. (2005). Effects of an EEG biofeedback protocol on a mixed substance abusing population. The American Journal of Drug and Alcohol Abuse, 31, 455-469. https://doi.org/10.1081/ada-200056807.
Weise, R., von Mengden, I., Glos, M., Garcia, C., & Penzel, T. (2013). P 153. Influence of transcranial slow oscillation current stimulation (tSOS) on EEG, sleepiness and alertness. Clinical Neurophysiology, 124(10), e137. https://doi.org/10.1016/j.clinph.2013.04.230
Selecting a training protocol model
This section covers qEEG-Based Neurofeedback Training, The Development of Z-Score Training, Infra-Low Training, Infra-Slow Training, and Connectivity Training.
qEEG-Based Neurofeedback Training
Following the development and proliferation of high-quality EEG recording devices capable of recording 19 scalp electrode locations simultaneously and software capable of creating images derived from the recorded data, the use of a quantitative approach to the EEG began to be more broadly utilized.
Caption: qEEG treatment of ADHD
The quantitative assessment of scalp electrical recordings was made possible by the development of the Fast Fourier Transformation (FFT) algorithm in 1965 (Cooley & Tukey, 1965; Dumermuth & Fluhler, 1967).
This computation method allowed for the deconstruction of the complex EEG information, consisting of multiple frequency components with different amplitudes and characteristics, into frequency and power spectral displays, initially as tables and later as representative values mapped onto topographic head maps (brain maps).
As inexpensive and powerful personal computers became more available in the 1990s, trainers and researchers could utilize these advanced computational methods and begin to identify the components associated with various conditions and disorders. This was facilitated by developing EEG normative databases by E. Roy John at New York University and Robert Thatcher and the University of Maryland, Frank Duffy of Harvard University, and others.
 |
 |
Caption: E. Roy John and Frank Duffy
The EEG has been shown to have strong stability and specificity across multiple ethnic and cultural groups (John, Ahn, & Prichep, 1980) and to be highly consistent in evaluations of test-retest reliability (Fein et al., 1983; Oken & Chiappa, 1988).
Because of this consistency and reliability, the quantitative assessment of the EEG (qEEG) and the availability of qEEG databases became useful clinical tools for individualized training protocol development. Each client could be assessed using multiple techniques, including a qEEG with normative database comparison.
qEEG methods include the ability to compare EEG recordings to reference database samples. Some reference groups are considered “normal,” while others used are a sample of patients that may not be considered a good reference since by definition they are seeking medical care assumably for brain-related conditions. Other reference groups consist of special operations military groups or sports teams, which are peer-reference groups for comparison.
Having a reference group allows for comparison statistics like z-scores. The neurofeedback software can permit any number of frequency bands or all frequencies to be modulated in the direction of a z-score of 0. Z-scores can represent frequency amplitude or coherence values. Graphic © Peter Hermes Furian/Shutetrstock.com.
LORETA-guided neurofeedback can also include z-score measures, but it offers an added advantage over surface EEG neurofeedback. LORETA-guided neurofeedback allows brain regions of interest (e.g., Brodmann areas) to be the target of the feedback. The only additional consideration is that LORETA calculations require at least 16 scalp sensor connections, whereas surface z-score training can use a single active channel location (e.g., CZ). Individual athletes may have a specific area of interest to enhance, which can be achieved with LORETA.
As described by Thatcher (1998), the value of the normative EEG database comparison included the ability to assess client electrophysiological metrics to help determine the potential basis for the client’s complaints. Using a normative EEG database also helped identify strengths and weaknesses of the client’s neurophysiology to design the optimal training regime and aid in evaluating results following a given intervention.

Caption: Robert Thatcher
Many trainers began to utilize these tools to determine the best training approach for their clients. If an area of the brain showed excess or deficient activity in one or more frequencies, that area would be targeted with uptraining or down-training methods to address those specific issues. This often led to the resolution of the client’s presenting complaints associated with these underlying patterns of dysregulation.
The benefits of these approaches for training protocol development were obvious. There was no need to guess at the location of training based upon an understanding of neuroanatomy or neurophysiology. There was no need to guess at the frequency to target in training and no need to guess at the direction of that training – whether to reward increases or decreases in a given frequency or set of frequencies. Finally, the training results were identifiable with follow-up qEEG assessment upon subsequent analysis.
As database-guided training progressed, a few difficulties emerged. One of the first and most often called out by neurologists (Nuwer & Coutin-Churchman, 2014) is the artifact problem in the EEG. Inexperienced practitioners who had little training in electroencephalography often made errors in data selection and rejection when evaluating an EEG for subsequent processing and database comparison. The presence of eye artifacts, including blink, lateral eye movement, eye flutter, and others, leads to an overabundance of delta frequency activity in frontal and lateral frontal EEG sensor locations compared to a normative database. Graphic © eegatlas-online.com.
This is due to the frequency of eye artifact (slow, in the 1-4 Hz range) and amplitude (generally quite high, in the 50 to 100 uV range or higher) that is thus in the same frequency as the delta frequency band but with much higher voltages than typical resting delta activity.
Several other artifacts produced additional false-positive findings in the database report. These include EMG (muscle activation) artifact that artificially elevates beta and fast or high beta frequencies, cable sway artifact (in the delta range), electrical “mains” artifact or electromagnetic frequency (EMF) artifact that increases fast beta findings as well as a wide variety of other artifacts. Please see the section on EEG artifacts.
The second major error was to train any finding that showed deviation from normal or typical values compared to a well-screened, typical subjects database.
As inexpensive and powerful personal computers became more available in the 1990s, trainers and researchers utilized these advanced computational methods and began to identify the components associated with various conditions and disorders. This was facilitated by the development of EEG normative databases developed by E. Roy John (NxLink, later known as BrainDX) at New York University, Robert Thatcher (NeuroGuide) at the University of Maryland, Frank Duffy of Harvard University (BEAM), Barry Sterman and David Kaiser (SKIL), and others. More recent additions have included the Human Brain Institute (HBI) database developed by Juri Kropotov and the iSyncBrain database developed by South Korean researchers (iMediSync).
In some cases, training an abnormal finding resulted in the client reporting worsening symptoms, the return of previously resolved symptoms and in some cases, the emergence of serious negative symptoms that were new to the client.
This led to the understanding that not all abnormal or out-of-range findings on a qEEG assessment were associated with pathology. Findings on qEEG assessments needed to be correlated with other assessment findings and client-reported symptoms. It became clear that some changes in the EEG were compensatory and reflected the system’s attempts to find balance or homeostasis. Removing these compensatory behaviors often resulted in the negative effects noted previously.
From this developing understanding grew an idea that training could address only the specific symptom or network that was most problematic for the client and that would have built-in mechanisms to correct for movement of EEG variables in undesirable directions. This has led to the creation of direct database training or z-score training.
The Development of Z-Score Training
Several companies proficvide z-score neurofeedback that ranges from just a few EEG channels to all 19 or more. Frequencies are trained toward a statistical normal range, generally +/- 1.5 SD. Several display and training interfaces exist from different equipment providers.
Caption: Live Z-Score Training with Z-Bars and Z-Maps Display
Subsequent advances resulted in 19-channel, real-time surface z-score training, 19-channel LORETA z-score training, and then using the LORETA source localization to provide z-score neurofeedback for selected Brodmann Areas.
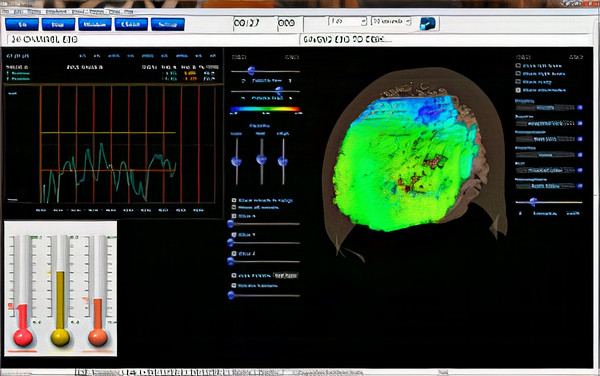
Caption: 19-Channel sLORETA Z-Score Training
Caption: 19-Channel Brodmann Area Z-Score Training
Glossary
ABA reversal design: a small N design where a baseline is followed by treatment and a return to baseline.
alpha blocking: the replacement of the alpha rhythm by low-amplitude desynchronized beta activity during movement, attention, mental effort like complex problem-solving, and visual processing.
alpha rhythm: 8-12-Hz activity that depends on the interaction between rhythmic burst firing by a subset of thalamocortical (TC) neurons linked by gap junctions and rhythmic inhibition by widely distributed reticular nucleus neurons. Researchers have correlated the alpha rhythm with relaxed wakefulness. Alpha is the dominant rhythm in adults and is located posteriorly. The alpha rhythm may be divided into alpha 1 (8-10 Hz) and alpha 2 (10-12 Hz).
alpha spindles: trains of alpha waves that are visible in the raw EEG and are observed during drowsiness, fatigue, and meditative practice.
amplitude: the strength of the EMG signal measured in microvolts or picowatts.
beta rhythm: 12-38-Hz activity associated with arousal and attention generated by brainstem mesencephalic reticular stimulation that depolarizes neurons in the thalamus and cortex. The beta rhythm can be divided into multiple ranges: beta 1 (12-15 Hz), beta 2 (15-18 Hz), beta 3 (18-25 Hz), and beta 4 (25-38 Hz).
beta spindles: trains of spindle-like waveforms with frequencies that can be lower than 20 Hz but more often fall between 22 and 25 Hz. They may signal ADHD, especially with tantrums, anxiety, autistic spectrum disorders (ASD), epilepsy, and insomnia.
bipolar (sequential) montage: a recording method that uses two active electrodes and a common reference.
common-mode rejection (CMR): the degree by which a differential amplifier boosts signal (differential gain) and artifact (common-mode gain).
delta rhythm: 0.05-3 Hz oscillations generated by thalamocortical neurons during stage 3 sleep.
desynchrony: pools of neurons fire independently due to stimulation of specific sensory pathways up to the midbrain and high-frequency stimulation of the reticular formation and nonspecific thalamic projection nuclei.
EEG activity: a single wave or series of waves.
epileptiform activity: spikes and sharp waves associated with seizure disorders.
Fast Fourier Transform (FFT): a mathematical transformation that converts a complex signal into component sine waves whose amplitude can be calculated.
frequency: the number of complete cycles that an AC signal completes in a second, usually expressed in hertz.
hertz (Hz): a unit of frequency measured in cycles per second.
infra-low-frequencies (ILF): frequencies below 0.1 Hz.
infra-slow-frequencies (ISF): frequencies below 0.1 Hz.
low resolution electromagnetic tomography (LORETA): Pascual-Marqui's (1994) mathematical inverse solution to identify the cortical sources of 19-electrode quantitative data acquired from the scalp.
posterior dominant rhythm (PDR): the highest-amplitude frequency detected at the posterior scalp when eyes are closed.
power: the amplitude squared and may be expressed as microvolts squared or picowatts/resistance.
protocol: a rigorously organized plan for training.
Quantitative EEG (qEEG): digitized statistical brain mapping using at least a 19-channel montage to measure EEG amplitude within specific frequency bins.
raw EEG signal: oscillating electrical potential differences detected from the scalp.
reference electrode: an electrode placed on the scalp, earlobe, or mastoid.
sampling rate: the number of times per second that an ADC samples the EEG signal.
sensorimotor rhythm (SMR): 13-15 Hz spindle-shaped sensorimotor rhythm (SMR) detected from the sensorimotor strip when individuals reduce attention to sensory input and reduce motor activity.
sleep spindles: waves that range from 12-15 Hz and last from 0.5 to several seconds widely distributed over the scalp and are observed during Stage 2 and 3 sleep.
standardized LORETA (sLORETA): a refinement of LORETA that estimates each voxel's electrical potentials without regard to their frequency, expresses normalized F-values, and achieves a 1-cubic-cm resolution.
surface Laplacian (SL) analysis: a family of mathematical algorithms that provide two-dimensional images of radial current flow from cortical dipoles to the scalp.
swLORETA: a more precise and accurate iteration of the LORETA source localization method.
theta/beta ratio (T/B ratio): the ratio between 4-7 Hz theta and 13-21 Hz beta, measured most typically along the midline and generally in the anterior midline near the 10-20 system location Fz.
theta rhythm: 4-8-Hz rhythms generated a cholinergic septohippocampal system that receives input from the ascending reticular formation and a noncholinergic system that originates in the entorhinal cortex, which corresponds to Brodmann areas 28 and 34 at the caudal region of the temporal lobe.
z-score training: neurofeedback protocol that reinforces in real-time closer approximations of client EEG values to those in a normative database.
References
Ayers, M. E. (1977). EEG Neurofeedback to bring individuals out of Level Two Coma [Abstract]. Biofeedback and Self-Regulation, 20(3), 304–305.
Berger, H (1929). Über das elektroenkephalogramm des menschen. Arch Psychiatr Nervenkr 87(1), 527–570.
Budzynski, T. H. (1977). Tuning in on the twilight zone. Psychology Today, 11, 38–44.
Budzynski, T. H. (1996). Brain brightening: Can neurofeedback improve cognitive process? Biofeedback, 24, 14–17.
Byers, A. P. (1998). Neurotherapy reference library (2nd ed.). Applied Psychophysiology and Biofeedback.
Evans, J. R., Dellinger, M. B., & Russell, H. L. (Eds.) (2020). Neurofeedback: The first fifty years. Academic Press.
Fehmi, L. G., & Robbins, J. (2008). Sweet surrender-Discovering the benefits of synchronous alpha brainwaves (pp. 231-254). Measuring the immeasurable: The scientific case for spirituality. Sounds True, Inc.
Fein, G., Galin, D., Johnstone, J., Yingling, C. D., Marcus, M., & Kiersch, M. E. (1983).EEG power spectra in normal and dyslexic children. I. Reliability during passive conditions. Electroencephalogr Clin Neurophysiol, 55(4), 399-405. https://doi.org/10.1016/0013-4694(83)90127-x
Hardt, J. V., & Kamiya, J. (1978). Anxiety change through electroencephalographic alpha feedback seen only in high anxiety subjects. Science, 201(4350).79-81. https://doi.org/10.1126/science.663641 PMID: 663641.
John, E. R., Ahn, H., & Prichep, L., Trepetin, M., Brown, D., & Kaye, H. (1980). Developmental equations for the electroencephalogram. Science, 210(4475), 1255-1258. https://doi.org/10.1126/science.7434026
Johnstone, J. & Gunkelman, J. (2003). Use of databases in QEEG evaluation. Journal of Neurotherapy, 7, 3-4, 31-52. https://doi.org/10.1300/J184v07n03_02
Kamiya, J. (1968). Conscious control of brain waves. Psychology Today, 1, 56-60.
Legarda, S. B., McMahon, D., Othmer, S., & Othmer S. (2011). Clinical neurofeedback: Case studies, proposed mechanism, and implications for pediatric neurology practice. J Child Neurol., 26(8),1045-1051. https://doi.org/10.1177/0883073811405052
Leong, S. L., Vanneste, S., Lim, J., Smith, M., Manning, P., & De Ridder, D. (2018). A randomised, double-blind, placebo-controlled parallel trial of closed-loop infraslow brain training in food addiction. Sci Rep 8(11659). https://doi.org/10.1038/s41598-018-30181-7
Lubar, J. F., & Bahler, W. W. (1976). Behavioral management of epileptic seizures following EEG biofeedback training of the sensorimotor rhythm. Biofeedback Self Regul, 1(1), 77-104. https://doi.org/10.1007/BF00998692. PMID: 825150.
Lubar, J. F., Shabsin, H. S, Natelson, S. E., Holdson, S. E., Holder, G. S., Whittsett, S. F., Pamplin, W. E., & Krulikowski, D. I. (1981). EEG operant conditioning in intractable epileptics. Archives of Neurology, 38, 700–704.
Lubar, J. F., & Shouse, M. N. (1976). EEG and behavioral changes in a hyperkinetic child concurrent with training of the sensorimotor rhythm (SMR): A preliminary report. Biofeedback Self Regul,1(3), 293-306. https://doi.org/10.1007/BF01001170. PMID: 990355.
Marzbani, H., Marateb, H. R., & Mansourian, M. (2016). Neurofeedback: A comprehensive review on system design, methodology and clinical applications. Basic and clinical neuroscience, 7(2), 143–158.
Monastra, V. J., Lubar, J. F., Linden, M., VanDeusen, P., Green, G., Wing, W., Phillips, A., & Fenger, T. N. (1999). Assessing attention deficit hyperactivity disorder via quantitative electroencephalography: An initial validation study. Neuropsychology, 13(3), 424–433. https://doi.org/10.1037/0894-4105.13.3.424
Nuwer, M. R., & Coutin-Churchman, P. (2014). Brain mapping and quantitative electroencephalogram. Encyclopedia of the Neurological Sciences (2nd ed.). Elsevier.
Oken, B. S., & Chiappa, K. H. (1988). Short-term variability in EEG frequency analysis. Electroencephalogr Clin Neurophysiol, 69, 191–198. https://doi.org/10.1016/0013-4694(88)90128-9
Peniston, E. G., & Kulkosky, P. J. (1989). Alpha-theta brain wave training and beta-endorphin levels in alcoholics. Alcoholism, Clinical and Experimental Research, 13, 271 – 279. https://doi.org/10.1111/J.1530-0277.1989.TB00325.X
Peniston, E. G., & Kulkosky, P. J. (1990). Alcoholic personality and alpha-theta brain wave training. Medical Psychotherapy, 3, 37–55.
Putman, J. A., Othmer, S. F., Othmer, S., & Pollock, V. E. (2005). TOVA results following inter-hemispheric bipolar EEG training. Journal of Neurotherapy: Investigations in Neuromodulation, Neurofeedback and Applied Neuroscience, 9(1), 37-52. https://doi.org/10.1300/J184v09n01_04
Ribas, V. R., Ribas, R., & Martins, H. (2016). The learning curve in neurofeedback of Peter Van Deusen: A review article. Dementia & Neuropsychologia, 10(2), 98–103. https://doi.org/10.1590/S1980-5764-2016DN1002005
Rogala, J., Jurewicz, K., Paluch, K., Kublik, E., Cetnarski, R., & Wróbel, A. (2016). The do's and don'ts of neurofeedback training: A review of the controlled studies using healthy adults. Frontiers in Human Neuroscience, 10, 301. https://doi.org/10.3389/fnhum.2016.00301
Shouse, M. N., & Lubar, J. F. (1979). Operant conditioning of EEG rhythms and ritalin in the treatment of hyperkinesis. Biofeedback and Self-Regulation, 4, 299–312. https://doi.org/10.1007/BF00998960
Sittenfeld, P., Budzynski, T., & Stoyva, J. (1976). Differential shaping of EEG theta rhythms. Biofeedback and Self-Regulation, 1, 31–45. https://doi.org/10.1007/BF00998689
Smith, M. L., Collura, T. F., Ferrara, J., & de Vries, J. (2014). Infra-slow fluctuation training in clinical practice: A technical history. NeuroRegulation, 1(2), 187–207. https://doi.org/10.15540/nr.1.2.187
Smith, M. L., Leiderman, L., & de Vries, J. (2017). Infra-slow fluctuation (ISF) for autism spectrum disorders. In T. F. Collura & J. A. Frederick (Eds.), Handbook of clinical QEEG and neurotherapy. Routledge Taylor and Francis Group.
Sterman, M. B. (1976). Effects of brain surgery and EEG operant conditioning on seizure latency following monomethylhydrazine in the cat. Exp. Neurol., 50, 757-765. https://doi.org/10.1016/0014-4886(76)90041-8
Sterman, M. B., & Friar, L. (1972). Suppression of seizures in an epileptic following sensorimotor EEG feedback training. Electroencephalogr Clin Neurophysiol,33(1), 89-95. https://doi.org/10.1016/0013-4694(72)90028-4. PMID: 4113278.
Sterman, M. B., Lopresti, R. W., & Fairchild, M. D. (1969). Electroencephalographic and behavioral studies of monomethylhydrazine toxicity in the cat. AMRL-TR-69-3, Aerospace Medical Research Laboratory, Air Force Systems Command, Wright-Patterson Air Force Base, Ohio.
Sterman, M. B., LoPresti, R. W., & Fairchild, M. D. (2010). Electroencephalographic and behavioral studies of monomethyl hydrazine toxicity in the cat. Journal of Neurotherapy, 14(4), 293-300, https://doi.org/10.1080/10874208.2010.523367
Strehl, U. (2014). What learning theories can teach us in designing neurofeedback treatments. Frontiers in Human Neuroscience, 8(894).
Swingle, P. (2014). Clinical versus normative databases: Case studies of clinical Q assessments. NeuroConnections.
Thatcher, R. W. (1998). Normative EEG databases and EEG biofeedback. Journal of Neurotherapy, 2(4), 8-39. https://doi.org/10.1300/J184v02n04_02
Thatcher, R. W., Lubar, J. F., & Koberda, J. L. (2019). Z-Score EEG biofeedback: Past, present, and future. Biofeedback, 47(4), 89–103. https://doi.org/10.5298/1081-5937-47.4.04
E. Introduction to SMR training
In the 1970s, Dr. Sterman demonstrated that epileptogenic EEG features were reduced by increasing 12-15Hz amplitude over the somatosensory motor cortex. This frequency range over this particular cortical region became known as the sensorimotor rhythm (SMR). Using the same application in the early 1980s, Dr. Lubar found that this neurofeedback protocol reduced excessive motor activity and increased attention among children diagnosed with ADHD. The mechanism of action appears multifactorial in how SMR relates to improved integration of cortical information processing and stability.

This section covers SMR Optimal Performance Applications.
SMR neurofeedback is applied to improve memory loss, anxiety, inattention, and insomnia. Dr. Thompson demonstrated that just one electrode at CZ with the SMR protocol could be effective and lasting in enhancing attention and improve academic performance.
The combination of cognitive, emotional, and psychophysiological benefits from neurofeedback improves performance. Faster reaction times have also been expressed as a reasonable outcome following SMR training, with implications shown for speed essential sports and those of fine motor precision (Hardt & Kamiya, 1978; Kouzak et al., 2018; Lubar & Lubar, 1984; Sterman, 1996; Sterman et al., 1970; Thompson & Thompson, 1998; Wilson et al., 2006).
Attention, emotions, and fine motor function are some of the variables related to high-performance shooting.
Shooters exposed to SMR neurofeedback training at locations C3, CZ, C4 performed better than those that received alpha enhancement training at location T3 and T4, band better than those that received no neurofeedback. (Gong et al., 2020). Graphic retrieved from https://www.firelinestc.com/courses-peak-performance.php.

Learning to experience an enhanced SMR pattern and then transferring this state to the field or shooting course is the generalization necessary to perform well. As seen with neurofeedback for golfers (Cheng, 2015), the same methods apply to shot placement with a pistol or rifle. SMR neurofeedback is linked to improved emotional and cognitive self-regulation and motor performance, both essential to master for elite shooting scenarios.
Muscle memory is important, of course, but so is performance “brain memory” – a shooter needs both.
References
Campos da Paz, V. K., Garcia, A., Campos da Paz Neto, A., & Tomaz, C. (2018). SMR neurofeedback training facilitates working memory performance in healthy older adults: A behavioral and EEG study. Frontiers in Behavioral Neuroscience, 12, 321. https://doi.org/10.3389/fnbeh.2018.00321
Cheng, M. Y., Huang, C. J., Chang, Y. K., Koester, D., Schack, T., & Hung, T. M. (2015). Sensorimotor rhythm neurofeedback enhances golf putting performance. Journal of Sport & Exercise Psychology, 37(6), 626–636. https://doi.org/10.1123/jsep.2015-0166
Doppelmayr, M., & Weber, E. (2011). Effects of SMR and theta/beta neurofeedback on reaction times, spatial abilities, and creativity. Journal of Neurotherapy, 15(2), 115-129. https://doi.org/10.1080/10874208.2011.570689
Gong, A., Nan, W., Yin, E., Jiang, C., & Fu, Y. (2020). Efficacy, trainability, and neuroplasticity of SMR vs alpha rhythm shooting performance neurofeedback training. Frontiers in Human Neuroscience, 14(94). https://dx.doi.org/10.3389%2Ffnhum.2020.00094
Hardt, J. V., & Kamiya, J. (1978). Anxiety change through electroencephalographic alpha feedback seen only in high anxiety subjects. Science, 201(4350), 79-81. https://doi.org/10.1126/science.663641 PMID: 663641.
Lubar, J. F., & Bahler, W. W. (1976). Behavioral management of epileptic seizures following EEG biofeedback training of the sensorimotor rhythm. Biofeedback Self Regul, 1(1), 77-104. https://doi.org/10.1007/BF00998692. PMID: 825150.
Lubar, J. O., & Lubar, J. F. (1984). Electroencephalographic biofeedback of SMR and beta for treatment of attention deficit disorders in a clinical setting. Biofeedback and Self-Regulation, 9(1), 1–23. https://doi.org/10.1007/BF00998842
Lubar, J. F., Shabsin, H. S, Natelson, S. E., Holdson, S. E., Holder, G. S., Whittsett, S. F., Pamplin, W. E., & Krulikowski, D. I. (1981). EEG operant conditioning in intractable epileptics. Archives of Neurology, 38, 700–704.
Lubar, J. F., & Shouse, M. N. (1976). EEG and behavioral changes in a hyperkinetic child concurrent with training of the sensorimotor rhythm (SMR): A preliminary report. Biofeedback Self Regul,1(3), 293-306. https://doi.org/10.1007/BF01001170. PMID: 990355.
Srinivasan, N. S. (2012). Enhancing neuroplasticity to improve peak performance. Biofeedback, 40(1), 30-33. https://doi.org/10.5298/1081-5937-40.1.2
Sterman, M. B. (1976). Effects of brain surgery and EEG operant conditioning on seizure latency following monomethylhydrazine in the cat. Exp. Neurol., 50, 757-765. https://doi.org/10.1016/0014-4886(76)90041-8
Sterman M. B. (1996). Physiological origins and functional correlates of EEG rhythmic activities: implications for self-regulation. Biofeedback and Self-Regulation, 21(1), 3–33. https://doi.org/10.1007/BF02214147
Sterman, M. B., & Friar, L. (1972). Suppression of seizures in an epileptic following sensorimotor EEG feedback training. Electroencephalogr Clin Neurophysiol,33(1), 89-95. https://doi.org/10.1016/0013-4694(72)90028-4. PMID: 4113278
Sterman, M. B., Howe, R. C., & Macdonald, L. R. (1970). Facilitation of spindle-burst sleep by conditioning of electroencephalographic activity while awake. Science, 167(3921), 1146–1148. https://doi.org/10.1126/science.167.3921.1146
Sterman, M. B., Lopresti, R. W., & Fairchild, M. D. (1969). Electroencephalographic and behavioral studies of monomethylhydrazine toxicity in the cat. AMRL-TR-69-3, Aerospace Medical Research Laboratory, Air Force Systems Command, Wright-Patterson Air Force Base, Ohio.
Sterman, M. B., LoPresti, R. W., & Fairchild, M. D. (2010). Electroencephalographic and behavioral studies of monomethyl hydrazine toxicity in the cat. Journal of Neurotherapy, 14(4), 293-300, https://doi.org/10.1080/10874208.2010.523367
Thompson, T., Steffert, T., Ros, T., Leach, J., & Gruzelier, J. (2008). EEG applications for sport and performance. Methods, 45(4), 279–288. https://doi.org/10.1016/j.ymeth.2008.07.006
Wilson, V., Peper, E., & Moss, D. (2006) “The Mind Room” in Italian soccer training: The use of biofeedback and neurofeedback for optimum performance. Biofeedback, 34, 79-81.
F. qEEG-BASED NFB IMPLEMENTATION (PROTOCOL DECISION MAKING)
Overview
This section presents a framework for deciding where to place electrodes for training and which EEG variable or variables to train. However, this framework is only intended to serve as a starting point for making training decisions. Practitioners should be prepared to account for their reasoning for selecting protocols. The framework described in this unit can provide the backdrop against which formulation and decision-making for neurofeedback take place.

This section covers the Fundamental Questions in Selecting a Protocol, Factors to Consider, Decision-Making and Ongoing Problem-Solving and Flowchart.
Fundamental Questions in Selecting a Protocol
The color bar legend helps determine at a glance the frequency and scalp location that is within our outside limits of the reference group.
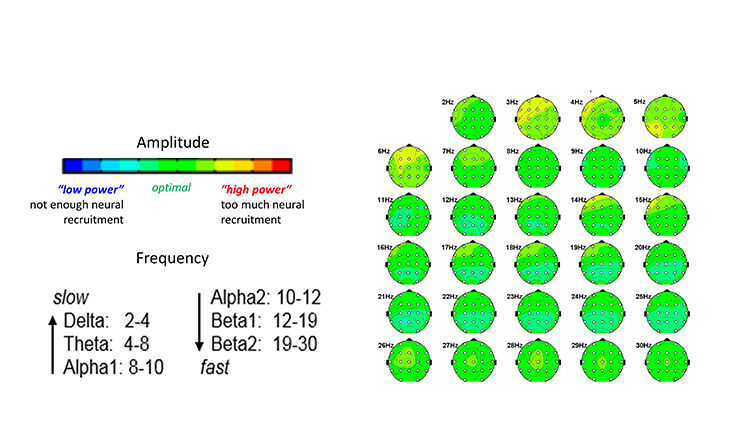
When considering a neurofeedback protocol for the individual, ask yourself three questions:
1. What frequency? (Slow or fast)
2. What power? (Low or high)
3. Which brain region.
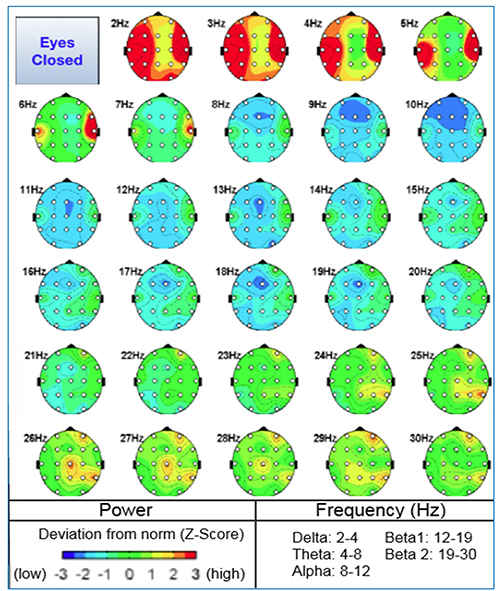
LORETA can aid in your decision to target regions of interest, either with Brodmann area neurofeedback targeting or with a surface single-channel. In this example, suppressing 5 Hz at Fz is a reasonable starting point to aid attention and focus. Reducing 6-7 Hz at P7 to assist language processing could be a secondary performance target.
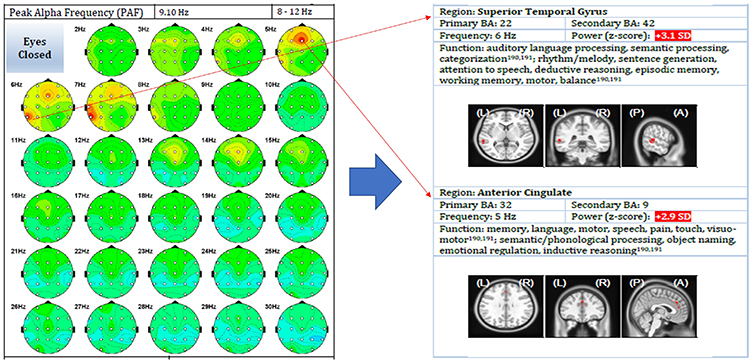
There are instances where a full array of 19 EEG sensors are needed to use LORETA Brodmann-specific targeting neurofeedback. In such a case, where a relative weakness is identified by qEEG analysis, the region of interest can be targeted for training.

In this example, the person benefited from training the Brodmann Area on the left hemisphere that, in part, was involved in ocular motor performance. She received visual positive feedback when the z-score was moving closer to zero. The decision to use this form of neurofeedback was based on the presenting problem and the ability to identify a region of interest.
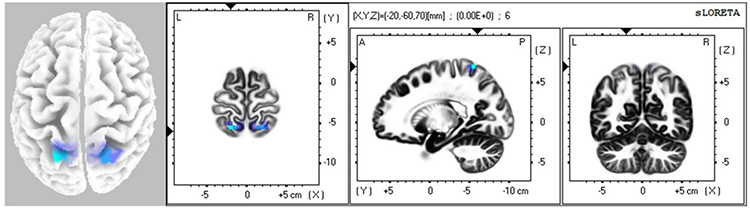
In other instances, the qEEG analysis offers patterns of function that would be readily amenable to a single-channel EEG sensor for amplitude training or even single or a few sensors using z-score neurofeedback training. Theta suppression neurofeedback at Fz would be a logical and straightforward application to aid focus and attention in the example.
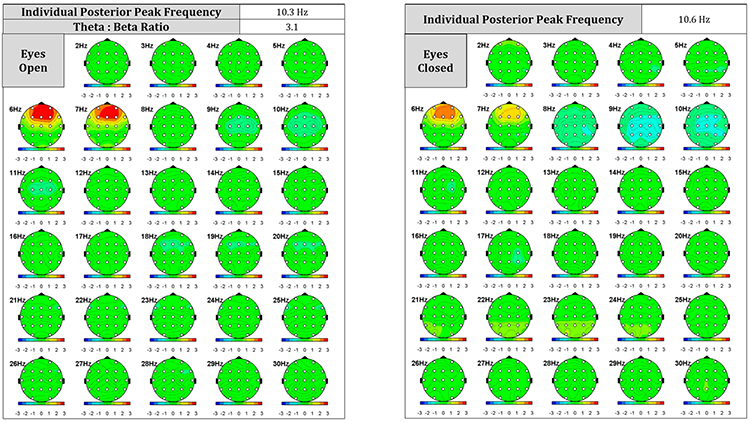
Developing Evidence-Based Treatment Protocols
We are indebted to the work of Michael and Lynda Thompson, who have worked with Vietta Sue Wilson and professional athletes to improve optimal performance using one channel EEG and a few channels of EEG with other biofeedback modalities. Using single EEG measures or even a series of bilateral sensor placements termed “miniQ” have a rich history in the neurofeedback literature and practice application(e.g., Thompson & Thompson, 2015).
Basic features of neurofeedback training protocols are electrode location, EEG frequency to be trained, EEG parameters (e.g., amplitude, amplitude ratios, connectivity measures, symmetry, peak frequency), and whether the EEG parameter for a frequency is trained up or down. When using 19-channel LORETA training, additional features to be considered for neurofeedback training protocols are cortical structures, Brodmann Areas, and brain networks. Graphic © Zayabich/Shutterstock.com. Graphic © Zayabich/Shutterstock.com.

Factors To Consider
Several factors are relevant to protocol selection, where to place electrodes, and which EEG variables to increase or decrease. These factors include the client’s condition, research findings, available resources, environmental context, client values and preferences, and performance targets.
Client Condition and Goals
Based on the initial assessment, the neurofeedback provider will know what the client’s goals are, that is, whether there are symptoms to decrease or abilities to increase. Also contributing to knowledge about the client’s condition are their personal and health histories and test findings. As described in the Assessment section of Neurofeedback Tutor: Assessment and Training, information relevant to this factor comes from various sources, including self-report, direct behavioral observation, professional reports, cognitive tests, questionnaires, and reports from significant others (e.g., parent, teacher, spouse). Results from EEG assessment are central to the client’s condition, and peripheral psychophysiological assessment findings are also considered in many cases.Training goals may be at one or more levels of analysis. One level is biological or the cortical location that helps identify the site(s) at which to train EEG variable like amplitude of a particular frequency. For example, the assessment finding of excess posterior beta activity in the parietal region in the context of a client's goal of racing thought reduction may suggest electrode placement at PZ
Another level of analysis has to do with socially significant behavior change in a specific context. For example, an assessment of a professional shooter with ADHD may show distractible behavior but can focus intently and perform well under shot pressure. The frontal midline theta excess seen on a QEEG map may or may not be the highest peak performance training priority. The person may have a greater need for fine motor stability and precision where a beta protocol over the motor cortex can help meet the performance needs.
Considering these levels of analysis in real-world contexts such as performance or mission-essential needs can be called a holistic biopsychosocial description of the client’s condition, which, together with knowledge from neuroscience research, leads to an individualized performance profile and plan. Information can also suggest where to place electrodes and what EEG variables to train.
Research Findings
Research findings to consider are of several types. One type is related to brain-behavior relationships and functional neuroanatomy. For example, language functions are a strength of the left hemisphere, and damage to the left temporal region may compromise language use. Graphic courtesy of Blausen.com staff "Blausen gallery 2014," Wikiversity Journal of Medicine.
A second type of research finding has to do with the functional significance of EEG findings and the correlation of various conditions with particular EEG signatures. For example, excess right frontal high beta and beta spindling have been associated with anxiety, and excess posterior beta has been related to anxiety. The movie below is a 19-channel display of low beta (13-21 Hz) and high beta (22-34 Hz) activity © John S. Anderson. Brighter colors represent higher beta amplitudes. Frequency histograms are displayed for each channel.
Third, anomalies in different brain networks are associated with various conditions and abilities (e.g., abnormality in default mode network functioning and depression). Neurofeedback training can increase or decrease connectivity using a normative database. For example, some neurofeedback software theoretically allows the training of brain regions associated with neuro-networks like the Default Mode Network (DMN). It is understood that networks are approximated and not as precisely targeted as marketing claims might suggest.
Last, neurofeedback efficacy research is essential to consider. For example, midline theta/beta training protocols have shown reliable efficacy in influencing the capacity to focus. Graphic © Oksana Mizina/Shutterstock.com.

With the description of the client’s performance needsand agreement on training goals, a good next step is to consider the degree to which research evidence shows benefit from any neurofeedback for goals that are similar to the individual with whom the neurofeedback provider is working.
The Efficacy unit of the companion Neurofeedback Tutor: An Introduction explained the scientific evidence levels and the differing levels of evidence available for various neurofeedback protocols for various client problems and goals. For some conditions, such as ADHD, there is a relatively large body of scientific evidence to support the efficacy or even effectiveness of neurofeedback.
Additionally, there may be more than one neurofeedback method that has been shown to have some degree of efficacy. With inattention, for example, theta/beta and SCP training along the midline have both been helpful. Therefore, one of these methods should be considered helpful to the client in this illustration.
As presented in the video for developing training protocols, a helpful strategy for the protocol section can be based on an arousal model that classifies client presentations into overaroused and underaroused. Research supports various protocols to address each of these two categories.
Selection of electrode site and EEG training parameters can also be based on an understanding of functional neuroanatomy, that is, the function of the cortical tissue beneath an electrode site or in a cortical region of interest or Brodmann Area, or the role of a particular location in brain networks that participate in particular functions or disorders. Researchers have revised the Brodmann maps and correlated areas with their functions. The Brodmann maps below were contributed by Mark Dow, Research Assistant at the Brain Development Lab, the University of Oregon, to Wikimedia Commons.

Selection is also based on normative values of frequency bands in particular locations and the understanding of whether excess values for EEG parameters may represent overarousal or underarousal in that area that could compromise a psychological or behavioral function.
Resources
The selection of training protocols depends on the software and hardware that the person has available. Such resources range from single-channel to 19-channel or more systems, with software capability ranging from amplitude and coherence training to the training of individual Brodmann Areas or approximating brain networks with z-score methods. Material resources for remote training may be an additional consideration in some cases. Staff resources may be an additional factor if training sessions need to be scheduled multiple times per week.Internal client resources may be psychological, such as motivation to change at this time, degree of self-awareness, communication, and cognitive abilities. Physical health should also be considered. For example, neurofeedback may be optimal when the client is ready and interested in training, can demonstrate some degree of self-awareness, can communicate at an age-appropriate level, and has stable physical and mental health and good sensory abilities.
Suppose the client has less than average psychological and physical resources. In that case, neurofeedback can be accommodated by several preliminary steps. These might include motivational interviewing to build readiness for change, peripheral biofeedback and self-monitoring to enhance self-awareness, matching verbal information to the client's ability level, and providing training when the client’s health is stable. In general, however, these factors may be of more importance to how a protocol is delivered than the protocol selection per se.
Peak Performance Trainer Expertise
Given the client’s condition and goal and the scientific evidence available to support neurofeedback training protocols for that condition and goal, the neurofeedback trainers consider whether their training, skills, experience, and equipment allow them to provide effective treatment.Evidence for neurofeedback applications to aid human performance is expanding each year. In what can be called an evidence-based model, which has its foundation in scientific investigations of neurofeedback efficacy and effectiveness. Evidence-Based Practice in Biofeedback and Neurofeedback (3rd ed.).

Additional to this foundation may be models of neurofeedback that are based on clinical studies and experience and the neuroscience of brain-behavior relationships. When research is unavailable to provide strong support for a specific neurofeedback protocol to address the athlete's condition or goals for improvement, the trainer must employ their knowledge of clinically-based models and neuroscience to aid in selecting a training plan.
Ethical principles are important. Trainers should provide neurofeedback only for conditions for which they have reasonable training or seek supervision. Ideally, the neurofeedback provider will have a sound understanding and experience with the condition and performance needs that the client presents, skill with neurofeedback methods for that condition, and knowledge of how that condition is related to the brain and psychophysiological findings. Graphic © WIN12_ET/Shutterstock.com.

Client Values and Preferences
The practitioner provides the client with a formulation that describes the problem, integrates the factors above into a provisional model that suggests the cause of the problem, and suggests training options. The options, with their likely costs and benefits, are reviewed with the client so that they can make an informed decision about which neurofeedback method to use or whether to pursue neurofeedback at all. This process recognizes the ethical principle of autonomy, the importance of informed consent, and acknowledges the client’s values and preferences. Graphic © ANDREI ASKIRKA/Shutterstock.com.
FROM ASSESSMENT TO TRAINING
The process of progressing through the assessment stage of working with a new client to the choice of protocol and training and then reassessment will be discussed through a series of client case examples.
Assessment has been covered in other sections within Neurofeedback Tutor. We begin with the client intake interview, which may have been facilitated by the client completing one or more history and symptom forms to guide the interview process. Once the client’s presenting concerns have been identified and discussed, the history of those concerns and whatever interventions have been tried can then be included to determine the previous level of treatment resistance and/or resolution experienced.
The client’s reasons for seeking neurofeedback training as a possible way to address these concerns can also be discussed. This is often an appropriate place to explain the underlying concepts of biofeedback training in general and neurofeedback training specifically. Particular emphasis on neurofeedback as training, not treatment, can be important at this stage of the developing client-trainer relationship.
Establishing the roles of both the client and the trainer is important to understand the training process. Many clients expect that neurofeedback and other types of biofeedback are interventions in the sense of something being done to the client, similar to muscle strengthening in the gym or receiving physical therapy.
Because all biofeedback involves training and education, this can be difficult for some clients to understand and accept. Biofeedback providers sometimes hear comments from clients such as “it didn’t work” or “that didn’t do anything.” This disconnect between the client’s expectations and the reality of the biofeedback training process may need to be addressed throughout the intervention.
Once the client understands the nature of the biofeedback training process, the trainer can proceed with whatever testing seems warranted, given the information obtained in the goal-setting interview and mission or athletic needs.
One of the most important aspects of the trainer relationship in biofeedback is the collaborative nature of the process. The trainer needs the client's active participation, and one of the goals is for the client to become a partner in the training process. The more informed and knowledgeable the client, the more likely they will give useful feedback about what they are experiencing and how the training affects them and their lives.
Re-evaluation is also an important part of the training process. A regular schedule of revisiting the initial assessment tools (i.e., 19-channel EEG, ERPs, ECG) can become an integral part of the training relationship. Ideally, the reassessment will guide protocol changes and help identify when the client has reached a plateau where no further change is likely.
Glossary
alpha asymmetry neurofeedback for mood disorders: a protocol that trains depressed clients to relax and warm their hands using diaphragmatic breathing and autogenic phrases and then decrease left frontal alpha with respect to the right frontal alpha.
alpha rhythm: 8-12-Hz activity that depends on the interaction between rhythmic burst firing by a subset of thalamocortical (TC) neurons linked by gap junctions and rhythmic inhibition by widely distributed reticular nucleus neurons. Researchers have correlated the alpha rhythm with "relaxed wakefulness." Alpha is the dominant rhythm in adults and is located posteriorly. The alpha rhythm may be divided into alpha 1 (8-10 Hz) and alpha 2 (10-12 Hz).
alpha spindles: regular bursts of alpha activity.
amplitude: the energy or power contained within the EEG signal measured in microvolts or picowatts.
beta rhythm: 12-38-Hz activity associated with arousal and attention generated by brainstem mesencephalic reticular stimulation that depolarizes neurons in both the thalamus and cortex. The beta rhythm can be divided into multiple ranges: beta 1 (12-15 Hz), beta 2 (15-18 Hz), beta 3 (18-25 Hz), and beta 4 (25-38 Hz).
beta spindles: trains of spindle-like waveforms with frequencies that can be lower than 20 Hz but more often fall between 22 and 25 Hz. They may signal ADHD, especially with tantrums, anxiety, autistic spectrum disorders (ASD), epilepsy, and insomnia.
Broca's area: the area located in the inferior frontal gyrus (BA 44 and 45) of the dominant hemisphere (F7-T3 in the left hemisphere) concerned with speech production, grammar, language comprehension, and sequencing.
Brodmann areas: 47 numbered cytoarchitectural zones of the cerebral cortex based on Nissl staining.
connectivity training: a strategy designed to correct deficient or excessive communication between two brain sites measured by indices like coherence and comodulation.
default mode network (DMN): a cortical network of sites located in frontal, temporal, and parietal regions that is most active during introspection and daydreaming and relatively inactive when we pursue external goals.
delta rhythm: 0.05-3-Hz oscillations generated by thalamocortical neurons during stage-3 sleep.
dominant frequency: the EEG frequency with the greatest amplitude.
evidence-based assessment: client evaluation using instruments that are reliable, valid, and possess clinical utility.
gamma rhythms: 28-80 Hz rhythm that includes the 38-42 Hz Sheer rhythm and is associated with learning and problem-solving, meditation, mental acuity, and peak brain function in children and adults.
hertz (Hz): unit of frequency, an abbreviation for cycles per second.
high alpha (alpha 2): 10-12-Hz alpha associated with open awareness.
high beta (beta 4): 25-38-Hz activity mostly seen in the frontal lobes and is associated with hyper-perfusion and increased glucose metabolism. High or fast beta activity may be related to peak performance and cognitive processing and related to specificity and precision in information processing. Excessive high beta is associated with alcoholism, anxiety, OCD, rumination, and worry.
local loops: neighboring cortical macrocolumns that share input generate frequencies above 30 Hz in the high-beta and gamma ranges.
local synchrony: synchrony that occurs when the coordinated firing of cortical neurons produces high-amplitude EEG signals.
low alpha (alpha 1): 8-10-Hz alpha below a client's peak alpha frequency when eyes are closed.
low resolution electromagnetic tomography (LORETA): Pascual-Marqui's (1994) mathematical inverse solution to identify the cortical sources of 19-electrode quantitative data acquired from the scalp.
module: a set of interconnected nodes in a neural network.
negative SCPs: slow cortical potentials produced by glial cells that increase the probability of neuron firing.
neural network: a system of interconnected ensembles of neurons that collaborate to achieve a goal. These networks communicate and perform functions via hub- or node-based communication systems.
node: vertex within a neural network.
normative database: means and standard deviations for EEG variables such as amplitude, power, coherence, and phase that are calculated for single hertz bins, frequency bands, or band ratios based on the EEG data collected from healthy normal subjects who are grouped by age, eyes-open or eyes-closed conditions, and sometimes gender and task, which also allows for the specification of z-scores with a mean of 0 and standard deviation of 1 for the various combinations of EEG variables, frequency ranges, subject ages, eyes open or eyes closed conditions, and other variables.
positive SCPs: slow cortical potentials produced by glial cells that decrease the probability of neuron firing.
quantitative EEG (qEEG): digitized statistical brain mapping using at least a 19-channel montage to measure EEG amplitude within specific frequency bins.
sensorimotor rhythm (SMR): 13-15 Hz spindle-shaped sensorimotor rhythm (SMR) detected from the sensorimotor strip when individuals reduce attention to sensory input and reduce motor activity.
slow cortical potential (SCP) training: neurofeedback to increase the gradual negative changes in the membrane potentials of cortical dendrites that last from 300 milliseconds to several seconds to reduce neuronal excitability in conditions like grand mal epilepsy and migraines.
slow cortical potentials (SCPs): gradual changes in the membrane potentials of cortical dendrites that last from 300 ms to several seconds. These potentials include the contingent negative variation (CNV), readiness potential, movement-related potentials (MRPs), and P300 and N400 potentials. SCPs modulate the firing rate of cortical pyramidal neurons by exciting or inhibiting their apical dendrites. They group the classical EEG rhythms using these synchronizing mechanisms.
swLORETA: a more precise and accurate iteration of the LORETA source localization method.
theta/beta ratio (T/B ratio): the ratio between 4-7 Hz theta and 13-21 Hz beta, measured most typically along the midline and generally in the anterior midline near the 10-20 system location Fz.
theta/beta training: a protocol that decreases theta amplitude and increases beta amplitude.
Wernicke's area: the area of the temporoparietal cortex (BA 22) of the dominant hemisphere specialized for speech perception and production. Damage can result in an inability to understand the meaning of speech and construct intelligible sentences.
z-score training: a strategy that attempts to normalize brain function with respect to mean values in a clinical database. EEG amplitudes that are 2 or more standard deviations above or below the database means are down-trained or uptrained to treat symptoms and improve performance.
References
Amzica, F., & Lopes da Silva, F. H. (2018). Cellular substrates of brain rhythms. In Schomer, D. L. & F. H. Lopes da Silva (Eds.). Niedermeyer's electroencephalography: Basic principles, clinical applications, and related fields (7th ed.). Oxford University Press.
Breedlove, S. M., & Watson, N. V. (2020). Behavioral neuroscience (9th ed.). Sinauer Associates, Inc.
Collura, T. F. (2014). Technical foundations of neurofeedback. Taylor & Francis.
Demos, J. N. (2019). Getting started with neurofeedback (2nd ed.). W. W. Norton & Company.
Gollan, J. K., Hoxha, D., Chihade, D., Pflieger, M. E., Rosebrock, L., & Cacioppo, J. (2014). Frontal alpha EEG asymmetry before and after behavioral activation treatment for depression. Biol Psychol, 99, 198-208. https://doi.org/10.1016/j.biopsycho.2014.03.003
Herbet, G., & Duffau, H. (2020). Revisiting the functional anatomy of the human brain: Toward a meta-networking theory of cerebral functions. Physiol Rev, 100, 1181-1128. https://doi.org/10.1152/physrev.00033.2019
Kaiser, D. A. (2008). Functional connectivity and aging: Comodulation and coherence differences. Journal of Neurotherapy, 12, 123-139. https://doi.org/10.1080/10874200802398790
LaVaque, T. J., Hammond, D. C., Trudeau, D., Monastra, V., Perry, J., Lehrer, P., Matheson, D., & Sherman, R. (2002). Template for developing guidelines for the evaluation of the clinical efficacy of psychophysiological evaluations. Applied Psychophysiology and Biofeedback, 27(4), 273-281.
Nunez, P. L., & Srinivasan, R. (2006). Electric fields of the brain (2nd ed.). Oxford University Press.
Swingle, P. G. (2015). Adding neurofeedback to your practice: trainer’s guide to ClinicalQ, neurofeedback, and braindriving. Springer.
Thatcher, R. W. (1998). Normative EEG databases and EEG biofeedback. Journal of Neurotherapy, 2(4), 8-39. https://doi.org/10.1300/J184v02n04_02
Thatcher, R. W., Lubar, J. F., & Koberda, J. L. (2019). Z-Score EEG biofeedback: Past, present, and future. Biofeedback, 47(4), 89–103. https://doi.org/10.5298/1081-5937-47.4.04
Thompson, M., & Thompson, L. (2015). Neurofeedback Book: An introduction to basic concepts in applied psychophysiology (second edition). Association for the Advancement of Psychophysiology and Biofeedback. https://doi.org/10.5298/1081-5937-44.1.09
G. Tan, F. Shaffer, R. R. Lyle, & I. Teo (Eds.). Evidence-based practice in biofeedback and neurofeedback (3rd ed.). Association for Applied Psychophysiology and Biofeedback.
G. REMOTE AND HOME-USE PERFORMANCE TRAINING
A growing number of limited scalp sensor devices for the recording of EEG are now available. Some have more consumer-facing simple use features, while others necessitate advanced understanding of EEG, frequency band selection, and scalp sensor placement. Some are laptop software-based and others rely on tablet or phone application software.
Some devices target specific functions (e.g., sleep) compatible with improved overall performance, such as improved sleep using SMR (12-15Hz enhance) at temporal scalp locations. It is important to recognize that anecdotal evidence is not the same as conclusive evidence. Additional controlled research remains an important industry objective (Lambert-Beaudet et al., 2021; Mirifar et al., 2017).

Other devices offer a home-use approach guided by a trained neurofeedback provider setting frequency bands, frequency power thresholds, and scalp placement locations. Several devices permit remote access and personalized neurofeedback protocol selection.

Advances in communications and computer technologies have made it possible for neurofeedback training to be more accessible so that clients and practitioners no longer must be in the same room. The ability to provide neurofeedback at a distance can significantly increase accessibility to training. However, many issues must be addressed to ensure that such training-at-a-distance, or remote neurofeedback training, is effective and safe. Graphic © Versus.


This section covers Considerations with Remote Training, Typical Course of Training, and Summary and Conclusions.
Considerations with Remote Training
Client-Related
Remote neurofeedback can increase the accessibility of training to a large number of clients in diverse locales. It may result in practitioners being able to serve more clients. Travel time and associated costs are reduced, and events that might lead to canceling an in-office session may be more easily accommodated by remote training. Remote training can also reduce exposure to infection and communicable diseases. Additionally, the outcome may be improved by delivering training in situations where change is most needed. Client preference may also be a deciding factor for training remotely. Graphic © Myndlift.
Typical Course of Training
If the considerations above suggest that remote training is desirable, the trainer will usually conduct an in-office assessment. Ideally, this is followed by a series of sessions in the trainer ’s office to ensure that the client responds well to training. The client and possibly a support person receive training to carry out the steps needed for remote training, and the necessary equipment and materials are acquired. Once remote training is begun, the trainer regularly reviews data with the client to track progress and make decisions about the next steps. The client may be asked to return to the office for re-assessment and adjustment of training parameters. Remote dashboards offer detailed data review and progress tracking, making periodic reviews convenient when clients are located at greater distances from the initial assessment and training location.
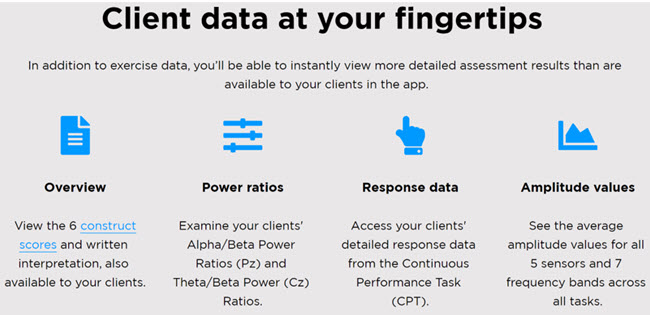
Summary and Conclusions
This section has reviewed the expanding practice of remote neurofeedback training. Such training presents several advantages but may not be possible in some situations due to client characteristics and resources. Before providing remote training, ethical, legal, and financial issues must be carefully considered. Because remote training has received such limited research attention, caution is necessary when offering it as an option to clients. Nevertheless, in many circumstances, the salient issues can be addressed, and the value of remote training can be significant.
Glossary
alpha rhythm: 8-12-Hz activity that depends on the interaction between rhythmic burst firing by a subset of thalamocortical (TC) neurons linked by gap junctions and rhythmic inhibition by widely distributed reticular nucleus neurons. Researchers have correlated the alpha rhythm with relaxed wakefulness. Alpha is the dominant rhythm in adults and is located posteriorly. The alpha rhythm may be divided into alpha 1 (8-10 Hz) and alpha 2 (10-12 Hz).
alpha-theta training: a protocol to slow the EEG to the 6-9 Hz crossover region while maintaining alertness.
amplitude: the strength of the EMG signal measured in microvolts or picowatts.
application (app): a specialized program downloaded onto a mobile device.
beta rhythm: 12-38-Hz activity associated with arousal and attention generated by brainstem mesencephalic reticular stimulation that depolarizes neurons in both the thalamus and cortex. The beta rhythm can be divided into multiple ranges: beta 1 (12-15 Hz), beta 2 (15-18 Hz), beta 3 (18-25 Hz), and beta 4 (25-38 Hz).
biofeedback-assisted relaxation (BART): the integration of biofeedback with relaxation procedures like autogenic training and progressive relaxation.
confidentiality: a client's right to keep personal information private.
delta rhythm: 0.05-3-Hz oscillations generated by thalamocortical neurons during stage 3 sleep.
EEG activity: a single wave or series of waves.
EMG biofeedback: the display of muscle action potentials detected by an electromyograph to a client.
frequency: the number of complete cycles that an AC signal completes in a second, usually expressed in hertz.
gamma rhythm: EEG activity frequencies above 30 or 35 Hz. Frequencies from 25-70 Hz are called low gamma, while those above 70 Hz represent high gamma.
heart rate (HR): the number of beats per minute.
heart rate variability (HRV): the organized fluctuation of time intervals between successive heartbeats defined as interbeat intervals.
hertz (Hz): the unit of frequency measured in cycles per second.
in-clinic BART: BART administered in a provider's office.
LORETA neurofeedback: low resolution electromagnetic tomography (LORETA). Pascual-Marqui's (1994) mathematical inverse solution to identify the cortical sources of 19-electrode quantitative data acquired from the scalp.
power: amplitude squared and may be expressed as microvolts squared or picowatts/resistance.
real time: as events occur.
remote HRV biofeedback: the delivery of heart rate variability biofeedback to a client who is in another physical location than the provider.
sensorimotor rhythm (SMR): 13-15-Hz spindle-shaped sensorimotor rhythm (SMR) detected from the sensorimotor strip when individuals reduce attention to sensory input and reduce motor activity.
theta/beta ratio: the ratio between 4-7 Hz theta and 13-21 Hz beta, measured most typically along the midline and generally in the anterior midline near the 10-20 system location Fz.
theta rhythm: 4-8-Hz rhythms generated a cholinergic septohippocampal system that receives input from the ascending reticular formation and a noncholinergic system that originates in the entorhinal cortex, which corresponds to Brodmann areas 28 and 34 at the caudal region of the temporal lobe.
References
Arena, J. G. (2010). Future directions in surface electromyography. Biofeedback, 38, 78-82. https://doi.org/10.5298/1081-5937-38.2.78
Association for Applied Psychophysiology and Biofeedback. (2016). Code of ethics. Retrieved October 31, 2021 from https://www.aapb.org/i4a/pages/index.cfm?pageid=3884
Biofeedback Certification International Alliance. (2016). Professional standards and ethical principles of biofeedback. https://bcia.memberclicks.net/assets/docs/ProfessionalStandardsAndEthicalPrinciplesof Biofeedback.pdf
Bioulac, S., Purper-Ouakil, D., Ros, T., Blasco-Fontecilla, H., Prats, M., Mayaud, L., & Brandeis, D. (2019). Personalized at-home neurofeedback compared with long-acting methylphenidate in an European non-inferiority randomized trial in children with ADHD. BMC Psychiatry, 19, 237-250. https://doi.org/10.1186/s12888-019-2218-0
BrainMaster Technologies Inc. (2020). Telehealth (Remote/Home Training). Retrieved on November 7, 2021 from https://www.youtube.com/watch?v=GFct171tAb0
BrainMaster Technologies Inc. Tele-health and remote training. Retrieved October 30, 2021 from https://brainmaster.com/tele-health/
College of Psychologists of Ontario. (2021). Virtual health care – enhanced privacy practices for services provided electronically. Retrieved October 30, 2021 from https://cpo.on.ca/virtual-health-care-enhanced-privacy-practices-for-services-provided-electronically-april-2021/
Brainworks. (2021). NeuroGuide hub and spoke training. Retrieved on November 7, 2021 from https://homeneurofeedback.com/fortherapists/
Cortoos, A., De Valck, E., Arns, M., Breteler, M. H. M., & Cluydts, R. (2010). An exploratory study on the effects of tele-neurofeedback and tele-biofeedback on objective and subjective sleep in patients with primary insomnia. Applied Psychophysiology and Biofeedback, 35, 125-134. https://doi.org/10.1007/s10484-009-9117-z
Davila, M. I., Kizakevich, P. N., Eckhoff, R., Morgan, J., Meleth, S., Ramirez, D., Nirgan, T., Strange, L. B., Lane, M., Weimer, B., Lewis, A., Lewis, G. F., & Hourani, L. I. (2021). Use of mobile technology paired with heart rate monitor to remotely quantify behavioral health markers among military reservists and first responders. Military Medicine, 186, 17-24. https://doi.org/10.1093/milmed/usaa395
Earles, J. E., Folen, R. A., & James, L. C. (2001). Biofeedback using telemedicine: Clinical applications and case illustrations. Behavioral Medicine, 27, 77-82. https://doi.org/10.1080/08964280109595774
Economides, M., Lehrer, P., Ranta, K., Nazander, A., Hilgert, O., Raevouri, A., Gevirtz, R., Khazan, I., & Forman-Hoffman, V. L. (2020). Feasibility and efficacy of the addition of heart rate variability biofeedback to a remote digital health intervention for depression. Applied Psychophysiology and Biofeedback, 45, 75-86. https://doi.org/10.1007/s10484-020-09458-z
Folen, R. A., James, L. C., Earles, J. E., & Andrasik, F. (2001). Biofeedback via telehealth: A new frontier for applied psychophysiology. Applied Psychophysiology and Biofeedback, 26, 195-204. https://doi.org/10.1023/A:1011346103638
Golebowicz, M., Levanon, Y., Palti, R., & Ratzon, N. Z. (2015). Efficacty of a telerehabilitation intervention programme using biofeedback among computer operators. Ergonomics, 58, 791-802. https://doi.org/10.1080/01140139.2014.982210
Hammond, D. C., Bodenhamer-Davis, G., Gluck, G., Stokes, D., Harper, S. H., Trudeau, D., MacDonald, M., Lunt, J., & Kirk, L. (2011). Standards of practice for neurofeedback and neurotherapy: A position paper of the International Society for Neurofeedback and Research. Journal of Neurotherapy, 15, 54-64. https://doi.org/10.1080/10874208.2010.545760
International Society for Neurofeedback and Research. (2020). Code of ethics. Retrieved October 31, 2021 from https://isnr.org/interested-professionals/isnr-code-of-ethics
Joint Task Force for the Development of Telepsychology Guidelines for Psychologists. (2013). Guidelines for the practice of telepsychology. American Psychologist, 68, 791-800. https://doi.org/10.1037/a0035001
Meru Health. (2021). Participant journey. Retrieved on November 7, 2021 from https://www.meruhealth.com/provider
Mirifar, A., Beckmann, J., & Ehrlenspiel, F. (2017). Neurofeedback as supplementary training for optimizing athletes' performance: A systematic review with implications for future research. Neurosci Biobehav Rev, 75, 419-432. https://doi.org/10.1016/j.neubiorev.2017.02.005
Schaefer, M., Iskander, J., Tams, S., & Butz, C. (2021). Offering biofeedback assisted relaxation training in a virtual world: Considerations and future directions. Clinical Practice in Pediatric Psychology, 9, 1-7. https://doi.org/10.1037/cpp0000391
Stetz, M. C., Folen, R. A., Van Horn, S., Ruseborn, D., & Samuel, K. M. (2013). Technology complementing military psychology programs and services in the Pacific Regional Medical Command. Psychological Services, 10, 283-285. https://doi.org/10.1037/a0027896
Versus. Mobile brain sensing. Retrieved October 30, 2021 from https://getversus.com/
Summary
The practitioner uses various methods during the intake assessment and organizes the resulting information into a summary that has utility for describing and understanding the client’s problems and goals and planning subsequent steps, including neurofeedback training. Data collected with various methods help the client make a confident and well-informed decision about whether to proceed with neurofeedback.
H. MONITORING TRAINING, MEASURING PROGRESS, AND GENERALIZATION TO FIELD/DUTY PERFORMANCE.
Overview

This section covers Continuous Performance Tasks, Abbreviated Q, and 19-Channel Recordings, Monitoring Training, and Measuring Progress.
Continuous Performance Tasks
Continuous performance tasks provide reaction time, processing speed, and response accuracy measures.
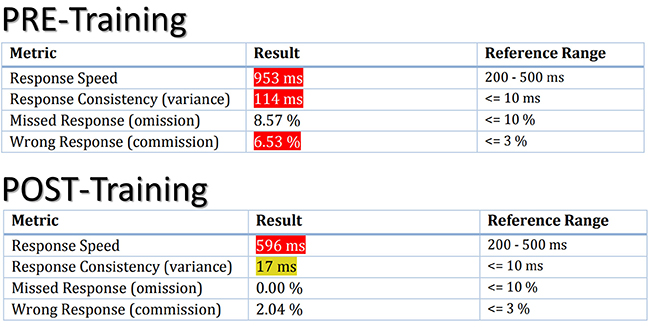
These are often recorded during Go-NoGo evoked potential evaluations that use midline EEG sensors or full-scalp sensor placements. Graphic retrieved from https://steamdb.info/app/923390/.

Speed of processing is important, but not necessarily at the expense of elevated errors. This couldn’t be more true than in military Shoot House training. Routine performance assessments will show areas of response function that could be a target for improvement. Graphic retrieved from https://actiontarget.com/categories/shoot-houses/.

Thalamic generated posterior alpha is typically faster than frontal alpha due to the more direct or faster thalamo-cortical-thalamic loop circuit. Image used by permission from David Hagedorn, PhD. Eyes-closed EEG showing an elevated peak alpha of a US Navy Corpsman following a kinetic deployment with a US Marine SOF.
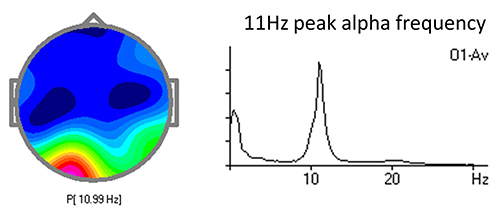
This pattern is amenable to alpha-theta crossover training with expected improved sleep, improved ability to relax, and potentially seek less personal use of alcohol -- all of which will improve cognitive and physical performance.
P300b change reflects 11% improvement in working memory. Pre-deployment measures can be readily referenced against post-deployment measures to measure cognitive degradation and target areas for peak performance training to return to baseline or better.
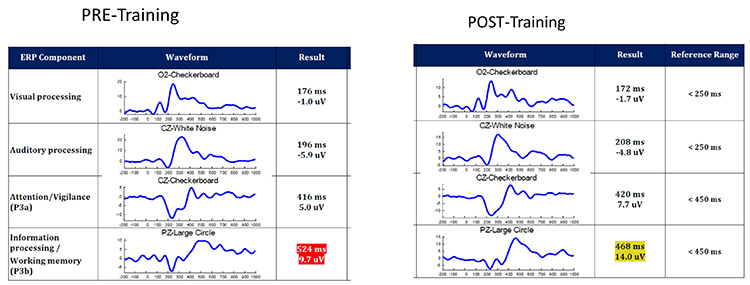
ABBREVIATED Q RECORDINGS
Perspective
Intake and initial EEG assessment provide a baseline against which subsequent progress, or lack of progress, can be gauged. This is important because, despite the robust research evidence for the efficacy and effectiveness of neurofeedback as shown in groups that receive training (Schwartz & Andrasik, 2016; Tan, Shaffer, Lyle, & Teo, 2016), benefits are not guaranteed for individual clients. One purpose of ongoing assessment is to verify that expected outcomes occur and, if a good result does not happen, use continuous assessment data to adjust training protocols.
Ethical principles involved in the ongoing assessment are related to questions of benevolence (i.e., is training helping?) and nonmalevolence (i.e., is training causing any harm?) (Beauchamp, 2003). Ongoing assessment is necessary to assure the former and avoid the latter, even if the harm is the continuing cost of time and finances for a treatment that does not help a particular individual. The ethical principle of autonomy involves whether the client wants to continue training. Ongoing assessment helps the client remain well-informed about the training outcome and can continue to provide consent to continue training. The ethical principle of justice may apply to neurofeedback training as training should be efficient (e.g., am I providing training efficiently enough to the current client that I do not unreasonably deny access to training to others?).
Additionally, ongoing assessment supports maintaining a positive working relationship by allowing the client and practitioner to examine outcomes and make evidence-based decisions collaboratively. Seeing progress through continuous assessment also motivates the client to persevere with training and apply skills that build on neurofeedback effects outside of the training session.
The process of reviewing data from ongoing assessment also may help the client to see relationships between their subjective states and behavior or even environmental events. For example, the client may develop a self-awareness that when the subjective state they experience during neurofeedback occurs in a given situation outside the training environment, they can behave more effectively. Such self-awareness can motivate the client to reproduce the subjective state they have intentionally learned with neurofeedback in advance of or during relevant situations.
Neurofeedback trains EEG activity of the brain, and ongoing assessment of EEG activity provides an index of whether neurofeedback has the intended effect on brain activity. However, the client and trainer are most likely interested in whether changes in EEG activity generalize to other domains such as peak performance, emotional experience, cognition, physiological condition, and real-life behavior in situations that matter to the client. Therefore, in addition to ongoing assessment of EEG activity, it is usually the case that the client and trainer will at least periodically use non-EEG measures during the ongoing evaluation. Therefore, continuous assessment typically includes using the same EEG, ERP, and ECG variables as those used during intake and repeating non-EEG measures used during the intake to assess the generalization of possible EEG changes to changes in those problems that originally motivated the client to request neurofeedback.
Measures and Methods
EEG Variables
A natural measure to use for ongoing assessment is the EEG variable (e.g., SMR power) that has been chosen for training, based on the hypothesis that neurofeedback should produce a change in that variable (e.g., rewarding SMR should lead to SMR increase). Recent research has shown that neurofeedback at a single site may also have effects that generalize to other sites and brain networks (e.g., Nicholson, Ros, Densmore, Frewen, et al., 2020). Therefore, practitioners who have access to 19-channel qEEG hardware and software may additionally be interested in assessing how single-channel changes may have generalized to changes in network function (Thatcher, 2020).The movie below is a 19-channel display of SMR activity © John S. Anderson. Brighter colors represent higher SMR amplitudes. Frequency histograms are displayed for each channel. Notice the runs of high-amplitude SMR activity.
The EEG variable targeted for training is usually measured during a pre-training baseline for each session. The saved data can be artifacted. The value of the EEG variable can then be graphed from session to session, with the value from the intake assessment being used as a baseline for these within-training session baselines (see Figure 1).
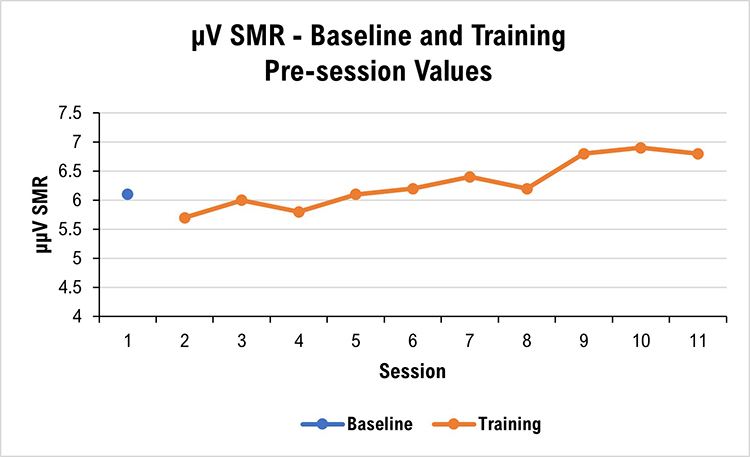
Many neurofeedback platforms make it easy to collect and graph EEG variables of interest, whether derived from single or multiple channels. Some platforms include a graphing capability, while the results of other platforms need to be copied and then pasted into a spreadsheet for graphing.
The EEG variable can also be measured again during a training session’s post-training baseline. This allows comparison with that session’s pre-training baseline and may show that the session’s training has produced a change in the intended direction. The pre-training and post-training baseline data from a series of sessions can be graphed separately or together, or a series of within-session change scores can be graphed (see Figure 2).
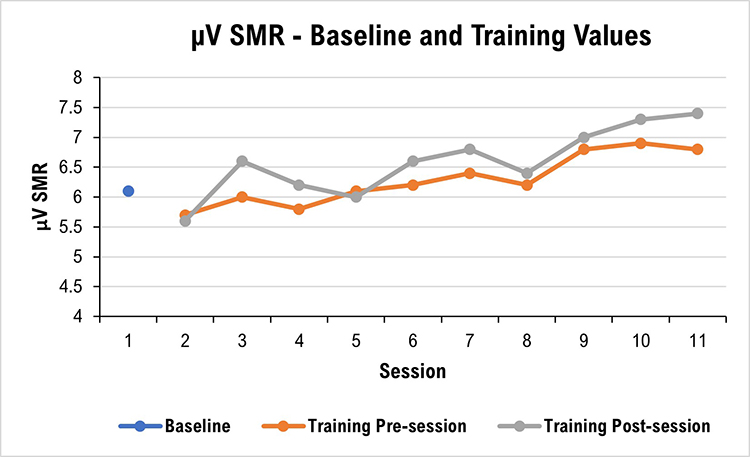
It can sometimes be interesting to graph EEG data from training blocks during a session. For example, if the client receives 10, 3-minute trials of neurofeedback in a session, the trainer can graph the series to show whether changes occur (see Figure 3).
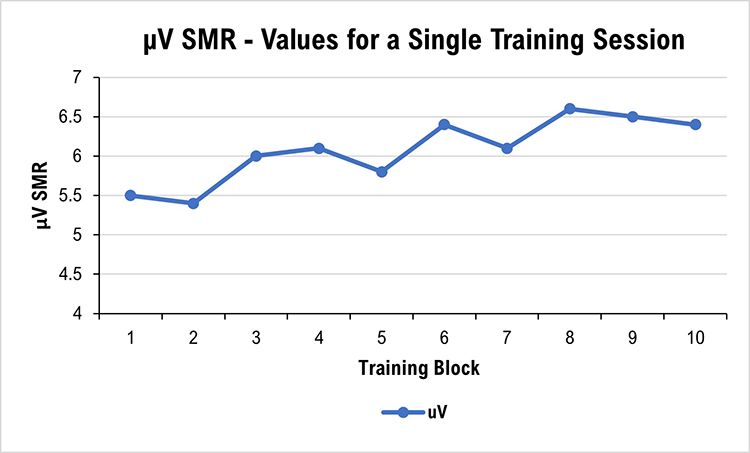
Qualitative Anecdotal Report
One of the products of intake assessment is the choice of a goal that has practical consequences for the client (e.g., feel less depressed, feel more relaxed, experience fewer headaches, concentrate better, study longer, avoid people less). Goals may be specified in positive or negative terms (e.g., more of A, or less of B) and refer to either internal states (e.g., thinking, emotion, somatic experience) or to behavior (e.g., number of pages written) (Hurn, Kneebone, & Cropley, 2006).Ongoing assessment of a qualitative nature can provide a detailed and informative description of how the client’s presenting problem changes during training. Anecdotal reporting invites the client to describe a personal situation or anecdote, together with the various dimensions of internal experience and behavioral performance, to represent the current state of their presenting problem or goal achievement.
At the beginning of each session, the neurofeedback provider can ask the client or a collateral informant to describe changes they are observing in the problem or goal for which they initially sought neurofeedback training. For example, both the child receiving neurofeedback and their parent can be asked how distractible the child has been during the past few days and their responses compared. Or, they could be asked to describe the situation in which they were most distractible to provide an anecdotal report linked to a specific problem at a particular time and place. Practitioner notes can be compared from session to session to give an impressionistic view of change.
Ongoing qualitative assessment, at least for the first few training sessions, is also helpful in monitoring for the possibility of unwanted side-effects of training (e.g., fatigue, excessive arousal, headache). Graphic courtesy of Migraine Buddy.

Quantitative Self-Report
Although such problems and goals may be expressed somewhat imprecisely and colloquially by clients, it can be helpful to define them more formally or quantitatively. Quantification makes it possible to graph progress over time and more easily see change (Greenhalgh & Meadows, 1999).Internal subjective states can be quantified with visual analog or Likert rating scales that label points on the scale. It is helpful to structure items with either Likert or visual analog scales (VAS). The rating can then be quantified and graphed for later review by the client and practitioner (Joshi et al., 2015; Wewers & Lowe, 1990). Definition of the scale item and labeling points on the scale help make the scale more reliable and valid as a measurement tool. At the beginning of a session, the client can mark their typical state during the previous few days or their state at the time of the session. VAS pain scale graphic © Overearth/Shutterstock.com.
Behavioral problems or goals can be operationally defined (i.e., in terms of the operations used to construct the definition; Martin & Pear, 2019). For example, suppose increased concentration (an internal state) is the goal. In that case, the duration of study time may be a useful behavioral goal to define in terms of minutes elapsed the previous day. At the beginning of a session, the neurofeedback provider can then ask the client to retrospectively estimate their study time during the day before the session.
One way of defining action-oriented or behavioral goals more formally is to use the SMART goals framework. SMART stands for specific, measurable, achievable, relevant, and time-bound (Bovend’Eerdt et al., 2009). Behavior analytic tactics (Cooper et al., 2007) can be used to address the specific, measurable, and time-bound components of SMART goals, for example, by defining goals in terms of frequency, intensity, and duration, and in terms of the context in which they should be met (e.g., during a typical week with ordinary stressors, a headache will occur not more than twice with an average duration of not more than 1 hour, and average severity of not more than 2 on a scale of 0 to 5). SMART goals graphic © Appleing/Shutterstock.com.
After intake assessment and definition of a SMART goal, the practitioner can quickly ask the client at the beginning of training sessions about the measurable feature of the SMART goal (e.g., for a socially anxious client, how many times did they speak at all to anyone besides the cashier during their most recent three trips for coffee?). Because the SMART goals are concrete and specific, the measurable rating that the client gives will tend to be reliable and, therefore, more valid than a response to a more open-ended question related to a general goal. Because a SMART goal can be easily quantified, their baseline and session-by-session values can be graphed for visual inspection and interpretation by the client and practitioner.
Self-Monitoring
Like the within-session reports described in the previous two sections, clients can be asked to make ratings at assigned times in assigned situations outside the neurofeedback session (e.g., at home after dinner, rate your level of calmness for the day taken as a whole). This type of self-monitoring of symptoms (e.g., stress, distractibility) or abilities (e.g., sustained attention, duration of time at a task, accuracy of task completion) relevant to the client’s presenting concerns can be started after intake assessment.Self-monitoring benefits the client by increasing their self-awareness in day-to-day situations and potentially strengthening their ability to self-regulate “internal” subjective experiences such as emotion, cognition, and tonic or reactive physiological states in service of effective behavior. Graphic courtesy of the Optimism family of mental health applications.
It can be useful to structure self-monitoring by identifying specific situations or times to use self-monitoring scales between training sessions. For example, the client can be asked to rate their concentration level during one or more particular classroom activities (e.g., completing algebra problems). Selecting a specific situation for rating helps to make the rating more reliable and valid by reducing variability due to differences among many situations.
It may be more relevant for other clients to use a time-based method for self-monitoring. The client can be asked, for example, to rate their level of calmness at the beginning of the day after their first hour of work or the end of the day based on their overall impression of the day as a whole. When self-monitoring between training sessions is used, it is essential to plan with the client to remember when to implement the measure. Specifically defined situational or temporal cues are effective in this regard. If self-monitoring in a particular situation is required, then verbal or visual reminders are helpful. For example, a post-it note in a notebook used for algebra can cue clients to rate their concentration level after class. Electronic alarms can also serve to prompt completion of the rating, and smartphone apps can be used in place of paper-and-pencil forms (Bakker & Rickard, 2018; Melbye et al., 2020).
Summary
This section has reviewed approaches to ongoing assessment and placed them within ethical and evidence-based health care frameworks. In addition, decision-making options have been suggested based on observed results of training.
Glossary
classical conditioning: unconscious associative learning process that builds connections between paired stimuli that follow each other in time.
collateral informant: an individual who can provide detailed background information or more accurate information regarding deviant behavior.
ongoing assessment: the continuous evaluation of client progress.
qualitative anecdotal report: a narrative description of changes in the problem or goal by the client or collateral informant.
quantitative self-report: a client's numerical ratings of symptoms, subjective states, and behaviors.
self-monitoring: a client's ratings at assigned times in specified situations outside the neurofeedback session to increaseself-awareness.
SMART: a framework that prescribes goals that are specific, measurable, achievable, relevant, and time-bound.
References
Baer, L., & Blais, M. A. (2010). (Eds.). Handbook of clinical rating scales and assessment in psychiatry and mental health. Humana Press.
Bakker, D., & Rickard, N. (2018). Engagement in mobile phone app for self-monitoring of emotional wellbeing predicts changes in mental health: MoodPrism. Journal of Affective Disorders, 227, 432-442. https://doi.org/10.1016/j.jad.2017.11.016
Beauchamp, T. L. (2003). Methods and principles in biomedical ethics. Journal of Medical Ethics, 29, 269-274. https://doi.org/10.1136/jme.29.5.269
Beck, A. T. (1996). Beck Depression Inventory – II. Psychological Corporation.
Bovend’Eerdt, T., J. H., Botell, R. E., & Wade, D. T. (2009). Writing SMART rehabilitation goals and achieving goal attainment scaling: A practical guide. Clinical Rehabilitation, 23, 352-361. https://doi.org/10.1177/0269215508101741
Cooper, J. O., Heron, T. E., & Heward, W. L. (2007). Applied behavior analysis (2nd ed.). Pearson Merrill Prentice Hall.
Gaultieri, T., & Johnson, L. G. (2006). Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Archives of Clinical Neuropsychology, 21, 623-643. https://doi.org/10.1016/j.acn.2006.05.007
Greenhalgh, J., & Meadows, K. (1999). Effectiveness of the use of patient-based measures of health in routine practice in improving the process and outcomes of patient care: A literature review. Journal of Evaluation in Clinical Practice, 5, 401-416. https://doi.org/0.1046/j.1365-2753.1999.00209.x
Hurn, J., Kneebone, I., & Cropley, M. (2006). Goal-setting as an outcome measure: A systematic review. Clinical Rehabilitation, 20, 756-72. https://doi.org/10.1177/0269215506070793
Joshi, A., Kale, S., Chandel, S., & Pal, D. K. (2015). Likert Scale: Explored and Explained. Current Journal of Applied Science and Technology, 7, 396-403.
Kamphaus, R., & Dever, B. V. (2016). Behavioral observations and assessment. In J. C. Norcross, G. R. VandenBos, D. K. Freedheim, & R. Krishnamurthy (Eds.), APA handbook of clinical psychology: Applications and methods (Vol. 3) (pp. 17-29). American Psychological Association.
Khazan, I. Z. (2013). Clinical handbook of biofeedback: A step-by-step guide for training and practice with mindfulness. Wiley-Blackwell.
Lambert-Beaudet, F., Journault, W. G., Rudziavic Provençal, A., & Bastien, C. H. (2021). Neurofeedback for insomnia: Current state of research. World Journal of Psychiatry, 11(10), 897–914. https://doi.org/10.5498/wjp.v11.i10.897
Lau, M. A., Bishop, S. R., Segal, Z. V., Buis, T., Anderson, N. D., Carlson, L., Shapiro, S., & Carmody, J. (2006). Toronto Mindfulness Scale: Development and validation. Journal of Clinical Psychology, 62, 1445-1467. https://doi.org/10.1002/jclp.20326
Lovibond, S. H. & Lovibond, P. F. (1995). Manual for the Depression Anxiety & Stress Scales (2nd Ed.). Psychology Foundation.
MacKillop, J., & Anderson, E. J. (2007). Further psychometric validation of the Mindful Attention Awareness Scale (MAAS). Journal of Psychopathology and Behavioral Assessment, 29, 289-293. https://doi.org/10.1007/s10862-007-9045-1
Martin, G., & Pear, J. J. (2019). Behavior modification: What it is and how to do it (11th ed.). Routledge.
McMaster University Health Sciences Library. (2021, May 5). https://hslmcmaster.libguides.com/c.php?g=306765&p=2044668
Melbye, S., Kessing, L. V., Bardram, J. E., & Fourholt-Jepsen, M. (2020). Smartphone-base self-monitoring, treatment, and automatically generated data in children, adolescents, and young adults with psychiatric disorders: Systematic review. JMIR Mental Health, 7, e17453.
Melnyk, B. M., Fineout-Overholt, E., Stillwell, S. B., & Williamson, K. M. (2010). Evidence-based practice step by step. American Journal of Nursing, 110, 51-53. https://doi.org/10.1097/01.NAJ.0000366056.06605.d2
Mirifar, A., Beckmann, J., & Ehrlenspiel, F. (2017). Neurofeedback as supplementary training for optimizing athletes' performance: A systematic review with implications for future research. Neuroscience and Biobehavioral Reviews, 75, 419–432. https://doi.org/10.1016/j.neubiorev.2017.02.005
Nicholson, A. A., Ros, T., Densmore, M., Frewen, P. A., Neufeld, R. W. J., Theberge, J., Jetly, R., & Lanius, R. A. (2020). Randomized, controlled trial of alpha-rhythm EEG neurofeedback in posttraumatic stress disorder: A preliminary investigation showing evidence of decreased PTSD symptoms and restored default mode and salience network connectivity using fMRI. NeuroImage: Clinical, 28, e102490. https://doi.org/10.1016/j.nicl.2020.102490
Randolph, C., Tierney, M. C., Mohr, E., & Chase, T. N. (1998). The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. Journal of Clinical and Experimental Neuropsychology. 20, 310–319. https://doi.org/10.1076/jcen.20.3.310.823
Schwartz, M. S., & Andrasik, F. (Eds.). (2016). Biofeedback: A practitioner’s guide (4th ed.). Guilford Press.
Tan, G., Shaffer, F., Lyle, R., & Teo, I. (2016). Evidence-based practice in biofeedback & neurofeedback (3rd Ed.). Association for Applied Psychophysiology and Biofeedback.
Tate, R. (2010). Compendium of tests, scales and questionnaires: Practitioner's guide to measuring outcomes after acquired brain impairment. Psychology Press.
Thatcher, R. W. (2020). Handbook of quantitative EEG and EEG biofeedback (2nd Ed.). ANI Publishing.
Thompson, M., & Thompson, L. (2015). Neurofeedback book: An introduction to basic concepts in applied psychophysiology (2nd ed.). Association for Advancement of Psychophysiology and Biofeedback.
Weiner, G. (2017). Evolving as a neurotherapist: Integrating psychotherapy and neurofeedback. In T. Collura and J. A. Frederick (Eds.), Handbook clinical QEEG and neurotherapy (pp. 45-54). Routledge.
Wewers, M. E., & Lowe, N. K. (1990). Critical review of visual analogue scales in the measurement of clinical phenomena. Research in Nursing and Health, 13, 227-236. https://doi.org/10.1002/nur.4770130405
I. FULL NEUROFEEDBACK SESSION DEMONSTRATIONS
Client education is the foundation of neurofeedback training (NFT). Professionals can explain core concepts, provide a road map of the NFT process, clarify their respective roles in the training process, and summarize training policies. The initial session provides an opportunity to address misconceptions about how NFT works and what the equipment does. Written informed consent is a contract that codifies the terms of your relationship with your client.
Applicants must develop competence in EEG equipment setup and operation. Demonstrations and hands-on training in didactic training programs provide a helpful introduction to instrumentation and software.
EEG equipment literacy requires that trainers understand how to measure the scalp, identify International 10-20 System sites, and attach electrodes. They must understand what a normal raw EEG looks like and gain experience in creating and controlling artifacts so that EEG measurements and brain maps based on them are valid. Finally, MCP trainers must learn to recognize some of the basics of abnormal EEG waveforms and distinguish them from benign activity.

This section covers the International 10-20 System, 19-channel EEG recording, NewQ Assessment with Live Demonstrations, and a Demonstration of a Full Neurofeedback Session.
International 10-20 System Demonstration
This demonstration covers the international 10-20 electrode placement system for EEG. It begins with an overview of graphic representations of the 19 scalp locations, the coordinate designations, and how the skull is measured to identify the correct locations. This is followed by live demonstrations of the basic measuring techniques and a standard sensor application for two separate EEG recording channels.
Video © J. S. Anderson.
FULL NEUROFEEDBACK TRAINING SESSION DEMONSTRATION
Session Description
The following video shows a demonstration of a basic neurofeedback session. This is a fictional example of session 11 in a sequence of training sessions with a fictional client, played by Cortney Amundson of Mindful Restoration, using a single EEG channel, training one location (Cz) in the center midline in a referential (monopolar) montage with an ear reference (Cz-A2). The training protocol involves rewarding increases in 12-15 Hz EEG activity – often called the sensorimotor rhythm or SMR – over the sensory/motor cortex while concurrently inhibiting 4-8 Hz theta and 22-36 Hz high or fast beta.The client (Sharon) relates her responses to the previous session, in this case, a negative reaction to a right-side training protocol (P4-T4 uptraining 8-12 Hz) that has shown positive responses in other clients. Also, the previous session included a short (5 minutes) training segment at Fp1, rewarding an increase in 15-18 Hz beta to address self-reported depressed mood. She reports a headache following the session and difficulty sleeping with gradual improvement until getting a good night’s sleep the night before this training session.
This is an example of the type of negative effects clients will sometimes experience from the training. This is not a typical occurrence, but neurofeedback providers need to be prepared often enough. The first thing to do is to accept that the client is relating an accurate experience. Validating the client and recognizing the potential for negative effects is important both for the client and the trainer. Too often, trainers will discount or reject the possibility of negative effects. This attitude is harmful to the training relationship as the client will be less likely to trust the provider if their experience is rejected or denied. This rejection of accurate reporting will also interfere with the trainer's ability to evaluate the training effect, particularly if the client becomes sensitized to only providing positive reports.
In the video, the trainer acknowledges the client’s report and suggests that the effect of one or both training protocols may have contributed to the negative effects and offers additional information about the possible effects, particularly the possibility of over-activation from the left frontal beta training. This elicits further information from the client that she “had more energy” following the training.
Finally, a return to the previous, successful Cz, SMR training approach is discussed. The possibility of shifting to direct z-score and database training is introduced as a possible future intervention. These brief comments and the discussion as a whole demonstrate the collaborative nature of the client–trainer relationship. Bidirectional communication and discussion are essential. Trainers must always remember that all biofeedback, including EEG biofeedback or neurofeedback, is training, not treatment.
The trainer fosters collaboration with the client to reach the client’s goals, which requires an egalitarian relationship rather than a top-down, practitioner-to-recipient approach. At its best, neurofeedback training involves client education regarding their nervous system while at the same time educating the nervous system directly. Ideally, the trainer also approaches the process from an educational perspective, learning from the client and observations of the process, being alert for mistakes (hopefully few), acknowledging those mistakes, and learning from them.
Relying on evidence-based training approaches helps minimize negative effects. This means that a thorough understanding of the research and performance literature is essential for the trainer to understand the expected effects of each type of training and what to do when unique, individual client responses occur. In the example in the video, the possible negative effects of left frontal beta training can include over activation of the central nervous system, possibly resulting in physiological symptoms of pain, headache, and sleep problems, as the client hasn’t learned to self-correct the overarousal condition. Further training in more rhythmic patterns such as SMR or alpha has been shown to produce a calming effect in most clients, as we see in this example in the video. This is just one example of the possible results of a training approach and a possible correction of that effect.
Note that the client felt like taking more deep breaths, leading to a tingling feeling in her body. This may reflect some overbreathing behavior and suggests further biofeedback training, specifically respiration and heart rate variability training (HRV), which would also provide the client with further resources for self-regulation if they experience states of CNS over-arousal in the future.
The suggestion to shift to z-score training may provide a better approach due to its self-corrective nature. When training in real-time to a normative database, a shift in any trained measures away from zero standard deviations (SD) indicates movement in an undesired direction. The feedback will immediately respond to this change by reducing the reward, encouraging a shift back towards typical functioning.
Feedback Approach
The feedback provided to the client was primarily proportional feedback with a single, event-related tone. This means that all the other feedback constantly changed in response to the client’s EEG activity. The flower opens and closes in direct response to the SMR amplitude. The flower opens more when SMR amplitude increases and closes when amplitude decreases. The music also follows a similar pattern, increasing in volume for increases in SMR amplitude and decreasing when amplitude decreases. The high beta inhibit is reflected in the sound of ocean waves in the background, which become louder when high beta amplitude decreases (inverse setting). This rewards a decrease in high beta amplitude without interrupting the training.When start/stop inhibit approaches are used, they can interrupt the training experience to a certain degree, particularly when doing more relaxation-oriented training. Research has shown better outcomes for biofeedback learning with proportional feedback than binary feedback (on/off) (Colgan, 1977; Strehl, 2014).
In this case, the single event-related or binary reward feedback signal is a "bong" sound that occurs each time the SMR amplitude exceeds the threshold for at least 250 ms and continues sounding as long as amplitude remains above the threshold. This rewards not only an increase in amplitude but reinforces the client’s ability to maintain longer bursts of 12-15 Hz activity above the threshold.
An additional feedback sound occurs when the 4-8 Hz theta amplitude exceeds the threshold. That activates the sound of birds chirping, which alerts the client to such increases, often associated with drifting attention. However, this feedback signal doesn’t stop or inhibit the other feedback signals and allows training to continue.
If this were a client with issues of under-arousal and inattention, other feedback approaches would be employed that would provide more structured, event-related feedback, with start and stop settings and other tools to encourage alertness and maintenance of attention. Also, more clearly defined goals, possibly with a “points” counter, may be employed to further encourage participation with the possibility of points accumulation leading to additional rewards. However, this fictional individual is overly attentive, anxious, driven, and achievement-oriented, so a more fluid and less goal-directed feedback approach is often more effective.
Because this is simply a demonstration video, it is truncated to show examples of the type of conversation between trainer and client that occurs at regular intervals during training. The trainer checks in with the client to determine whether the training has the desired effect. Other measures are often monitored concurrently, and examples of such monitoring include peripheral skin temperature and galvanic skin response (GSR). These measures can alert the trainer to signs of distress in the client such as a decrease in finger temperature or an increase in sweat on the palm, prompting a check-in to determine the cause of such distress. The trainer also monitors the high beta and theta signals to determine if there is an increase in arousal, possibly reflecting anxiety, or a decrease in arousal, possibly reflecting fatigue or a lapse in attention, again prompting a check-in with the client.
Working with an experienced client, who understands the feedback and has learned to report symptom changes during the session, it is often not necessary to interrupt the training with conversation or check in with the client. This also promotes independence and individual responsibility on the client's part and reinforces the self-training nature of the process.
There would likely be more discussion of emotional/psychological material during an actual clinical session. There would also likely be more questions from the client about why the negative effects occurred. The client’s life experiences would be discussed, such as any changes in relationships, feedback from family members, friends and/or coworkers, and their general energy, mood, and other perceptions. For brevity, these types of discussions were not included in the video, although the between-session tracking questionnaire the client completed between each session is included below.
There are many between-session symptom tracking methods. Some clinics set up online tracking forms, and although this can introduce security and privacy issues, it is often more convenient for the client and the practitioner. Some tracking forms are highly structured and detailed. The one below is intentionally limited to encourage clients to use their own words to describe their responses. The trainer will then probe for more detailed information during the session. This type of form is also more likely to be completed by most clients, whereas some more involved forms will not be completed because they take more time than many clients are willing to devote to the process. Some trainers will have a range of forms to fit the client.

Video © J. S. Anderson.
CONCLUSION
This client example demonstrates a basic training session utilizing a simple intervention that has a long history of use in the field of neurofeedback and which has been the subject of several published studies (Arns et al., 2009; Campos da Paz et al., 2018; Gevensleben et al., 2013; Mohammadi et al., 2015; Rajabi et al., 2019). These studies have demonstrated improved results on various measures in different populations, from children to older adults. Research models have included comparisons with known efficacious interventions such as medication management of ADHD, sham feedback, and general outcome measures studies.
In the demonstration, the client reports “feeling more relaxed.” This subjective self-report corresponds with the results shown below that indicate an increase in SMR voltage across the session, a decrease in EMG artifact and fast beta activity, and a stable or slightly decreasing value for the theta inhibit.
The images below show the results of the training session in graph form with explanations below each image.
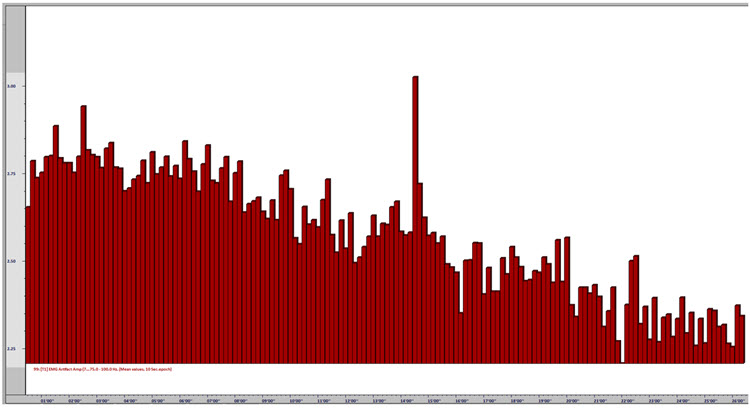
Caption: This image shows the EMG artifact (75-100 Hz) amplitude decreasing across the session. The y-axis represents voltage, and the x-axis represents time (approximately 26 minutes). The EMG voltage (muscle activity) decreases. Though the change is moderate, at .5 μV, it represents an approximately 20% decrease in EMG artifact across the session, associated with steadily increasing relaxation.
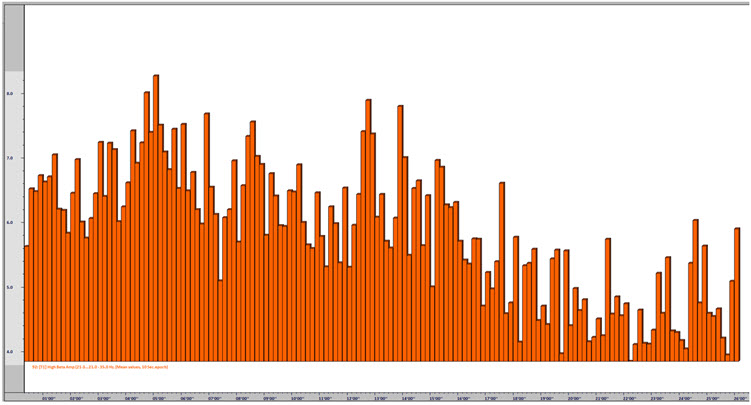
Caption: This image shows high beta (22-36 Hz) amplitude decreasing across the session. The y-axis represents voltage, and the x-axis represents time (approximately 26 minutes). High beta voltage decreases. Though the change is partially due to the decrease in EMG artifact (because of the impact of EMG activity on the beta frequencies), this change is more significant, showing initial values in the 6-8 μV range decreasing into the 4-5 μV range (discounting movement artifact). This change represents a significant decrease in fast beta activity and shows decreased cognitive arousal often related to worry and rumination.
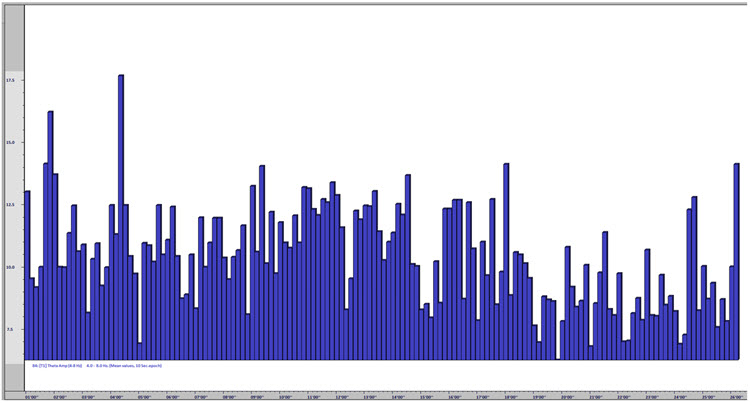
Caption: This image shows that theta (4-8 Hz) amplitude remained fairly steady across the session, with a moderate decrease toward the latter third of the session. The y-axis represents voltage, and the x-axis represents time (approximately 26 minutes). Although there is clear evidence of movement artifact and the likely influence of eye blink and eye movement artifact (this is an eyes-open training session, and hence there is more eye-related artifact influencing the slower frequencies), the session graph shows consistent values, with some change toward the end that may also be associated with the EMG reduction noted earlier.
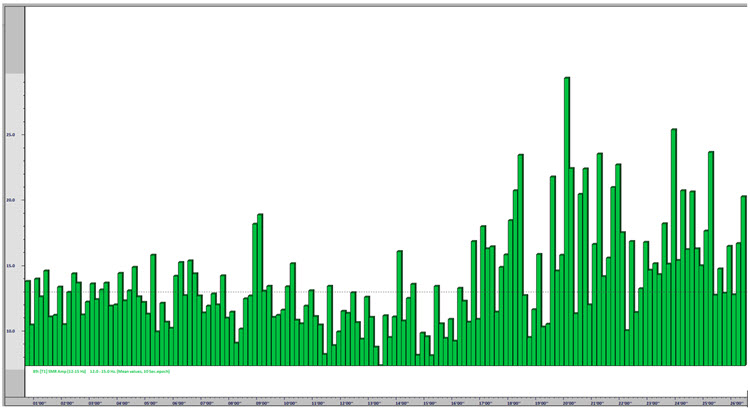
Caption: This image shows the SMR (12-15 Hz) amplitude increasing toward the end of the session. The y-axis represents voltage, and the x-axis represents time (approximately 26 minutes). This graph shows an interesting change in SMR that corresponds to the timing of the decrease in EMG artifact and high beta. The SMR amplitude increases at about the same time as these decreases, and this tells us two things: first, the increase in SMR amplitude is not likely to be the result of EMG artifact affecting the voltage of SMR, and second, the change in the state represented by all signals is consistently toward greater physiological relaxation and a decrease in CNS arousal.
Note the horizontal dotted line that represents the training threshold during the session (related to the “bong” reward sound for SMR above the threshold for 250 ms or longer) and note that the SMR amplitude is sustained above the threshold more consistently toward the end of the session, rewarding the client for this change in behavior and providing her with information validating her successful response to the training challenge.
Refer to the discussion on auto-threshold settings. If this session had been conducted with auto-threshold settings, the client would have received a constant positive reward at a steady percentage, resulting in no increase in reward percentage corresponding to her improved values toward the end of the session. Because the threshold for the SMR discrete, event-related reward remained the same throughout, the client could perceive her success by the increased frequency of the ‘bong’ sound as the session progressed.
As mentioned above, clients with inattentive ADHD would need a different approach. The session might be divided into 3-5 minute segments with pauses between segments to evaluate the client’s progress, discuss training strategies, adjust thresholds, or simply give the client a break rather than expecting sustained attention for the entire session. With other presenting concerns such as migraine, other clients would require a completely different approach, possibly with frequent adjustments of the training reward and inhibit frequencies, regular check-ins with the client to determine pain levels, and possible shifts in sensor locations.
There is no one-size-fits-all approach to neurofeedback training because each client is unique and requires interventions tailored to their presenting concerns and possible protocol adjustments based on their reactions to the training. This has been an example of one type of training approach with an adult client. Children and adolescents will present additional challenges for the practitioner and require patience, creativity, and attention.
Glossary
A (auricular): International 10-20 system earlobe reference placement.
alpha rhythm: 8-12-Hz activity that depends on the interaction between rhythmic burst firing by a subset of thalamocortical (TC) neurons linked by gap junctions and rhythmic inhibition by widely distributed reticular nucleus neurons. Researchers have correlated the alpha rhythm with relaxed wakefulness. Alpha is the dominant rhythm in adults and is located posteriorly. The alpha rhythm may be divided into alpha 1 (8-10 Hz) and alpha 2 (10-12 Hz).
amplitude: the strength of the EEG signal measured in microvolt or picowatts.
artifact: false signals like 50/60Hz noise produced by line current.
beta rhythm: 12-38-Hz activity associated with arousal and attention generated by brainstem mesencephalic reticular stimulation that depolarizes neurons in the thalamus and cortex. The beta rhythm can be divided into multiple ranges: beta 1 (12-15 Hz), beta 2 (15-18 Hz), beta 3 (18-25 Hz), and beta 4 (25-38 Hz).
bipolar (sequential) montage: a recording method that uses two active electrodes and a common reference.
C (central): sites in the International 10-20 system that detect frontal, parietal-occipital, and temporal EEG activity.
channel: EEG amplifier input from three leads (active, reference, and ground electrodes) placed on the head.
delta rhythm: 0.05-3 Hz oscillations generated by thalamocortical neurons during stage 3 sleep.
F (frontal): sites in the International 10-20 system that detect frontal lobe EEG activity.
Fp (frontopolar or prefrontal): sites in the International 10-20 system that detect prefrontal cortical EEG activity.
hertz (Hz): a unit of frequency measured in cycles per second.
impedance (Z): the complex opposition to an AC signal measured in Kohms.
impedance meter: a device that uses an AC signal to measure impedance in an electric circuit, such as between active and reference electrodes.
impedance test: automated or manual measurement of skin-electrode impedance.
inhibit training: setting a threshold to decrease unwanted EEG activity (e.g., 4-8 Hz and 22-36 Hz).
inion: a bony prominence on the back of the skull.
International 10-20 system: a standardized procedure for 21 recording and one ground electrode on adults.
mastoid bone: bony prominence behind the ear.
microvolt (μV): a unit of amplitude (signal strength) that is one-millionth of a volt.
referential (monoplar) montage: the placement of one active electrode (A) on the scalp and a neutral reference (R) and ground (G) on the ear or mastoid.
montage: a grouping of electrodes (combining derivations) to record EEG activity.
nasion: the depression at the bridge of the nose.
normative database: qEEG metrics obtained from a representative sample of participants during resting and active-task conditions.
notch filter: a filter that suppresses a narrow band of frequencies, such as those produced by line current at 50/60Hz.
O (occipital): sites in the International 10-20 system that detect occipital lobe EEG activity.
ohm (Ω): a unit of impedance or resistance.
P (parietal): sites in the International 10-20 system that detect parietal lobe EEG activity.
posterior dominant rhythm (PDR): the highest-amplitude frequency detected at the posterior scalp when eyes are closed.
power: the amplitude squared and may be expressed as microvolts squared or picowatts/resistance.
preauricular point: the slight depression located in front of the ear and above the earlobe.
protocol: a rigorously organized plan for training.
reference electrode: an electrode placed on the scalp, earlobe, or mastoid.
rhythmic temporal theta of drowsiness (RMTD): bitemporal left is greater than right in this longitudinal bipolar montage. Noted are notched rhythmic waveforms localized to the temporal regions, some of which are sharply contoured.
sensorimotor rhythm (SMR): the 13-15 Hz spindle-shaped sensorimotor rhythm (SMR) detected from the sensorimotor strip when individuals reduce attention to sensory input and reduce motor activity.
theta/beta ratio (T/B ratio): the ratio between 4-7 Hz theta and 13-21 Hz beta, measured most typically along the midline and generally in the anterior midline near the 10-20 system location Fz.
theta rhythm: 4-8-Hz rhythms generated a cholinergic septohippocampal system that receives input from the ascending reticular formation and a noncholinergic system that originates in the entorhinal cortex, which corresponds to Brodmann areas 28 and 34 at the caudal region of the temporal lobe.
z-score training: a neurofeedback protocol that reinforces in real-time closer approximations of client EEG values to those in a normative database.
tragus: the flap at the opening of the ear.
transient: isolated waveforms or complexes that can be distinguished from background activity.
vertex (Cz): the intersection of imaginary lines drawn from the nasion to inion and between the two preauricular points in the International 10-10 and 10-20 systems.
Test Yourself
Customers enrolled on the ClassMarker platform should click on its logo to take 10-question tests over this unit (no exam password).

Appreciation
Dr. David Hagedorn generously contributed to the development of this unit. This content is used with permission.
References
Arns, M., de Ridder, S., Strehl, U., Breteler, M., & Coenen, A. (2009). Efficacy of neurofeedback treatment in ADHD: The effects on inattention, impulsivity and hyperactivity: A meta-analysis. Clinical EEG and Neuroscience, 40(3), 180–189. https://doi.org/10.1177/155005940904000311
Campos da Paz, V. K., Garcia, A., Campos da Paz Neto, A., & Tomaz, C. (2018). SMR neurofeedback training facilitates working memory performance in healthy older adults: A behavioral and EEG study. Frontiers in behavioral neuroscience, 12, 321. https://doi.org/10.3389/fnbeh.2018.00321
Colgan M. (1977). Effects of binary and proportional feedback on bidirectional control of heart rate. Psychophysiology, 14(2), 187–191. https://doi.org/10.1111/j.1469-8986.1977.tb03374.x
Gevensleben, H., Kleemeyer, M., Rothenberger, L. G., Studer, P., Flaig-Röhr, A., Moll, G. H., Rothenberger, A., & Heinrich, H. (2014). Neurofeedback in ADHD: Further pieces of the puzzle. Brain topography, 27(1), 20–32. https://doi.org/10.1007/s10548-013-0285-y
Mohammadi, M. R., Malmir, N., Khaleghi, A., & Aminiorani, M. (2015). Comparison of sensorimotor rhythm (SMR) and beta training on selective attention and symptoms in children with Attention Deficit/Hyperactivity Disorder (ADHD): A trend report. Iran J Psychiatry, 10(3), 165-174. PMCID: PMC4749686
Rajabi, S., Pakize, A., & Moradi, N. (2020). Effect of combined neurofeedback and game-based cognitive training on the treatment of ADHD: A randomized controlled study. Applied Neuropsychology Child, 9(3), 193–205. https://doi.org/10.1080/21622965.2018.1556101
Soutar, R., & Longo, R. (2022). Doing neurofeedback: An introduction (2nd ed.). ISNR Research Foundation.
Strehl U. (2014). What learning theories can teach us in designing neurofeedback treatments. Frontiers in Human Neuroscience, 8, 894. https://doi.org/10.3389/fnhum.2014.00894
Swingle, P. (2014). Clinical versus normative databases: Case studies of clinical Q assessments. NeuroConnections.
Thatcher, R. W. (1998). Normative EEG databases and EEG biofeedback. Journal of Neurotherapy, 2(4), 8-39. https://doi.org/10.1300/J184v02n04_02
Thatcher, R. W., Lubar, J. F., & Koberda, J. L. (2019). Z-Score EEG biofeedback: Past, present, and future. Biofeedback, 47(4), 89–103. https://doi.org/10.5298/1081-5937-47.4.04
Thomas, C. (2007). What is a montage? EEG instrumentation. American Society of Electroneurodiagnostic Technologists, Inc.
Thompson, M., & Thompson, L. (2015). Neurofeedback Book: An introduction to basic concepts in applied psychophysiology (2nd ed.). Association for the Advancement of Psychophysiology and Biofeedback.
